Structure-Property Relationships in Unsymmetric Bis(antiaromatics): Who Wins the Battle between Pentalene and Benzocyclobutadiene?†
- PMID: 32189503
- PMCID: PMC7311060
- DOI: 10.1021/acs.joc.9b03119
Structure-Property Relationships in Unsymmetric Bis(antiaromatics): Who Wins the Battle between Pentalene and Benzocyclobutadiene?†
Abstract
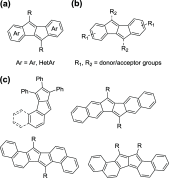
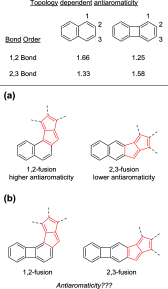
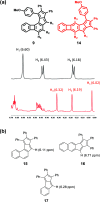
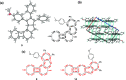
According to the currently accepted structure-property relationships, aceno-pentalenes with an angular shape (fused to the 1,2-bond of the acene) exhibit higher antiaromaticity than those with a linear shape (fused to the 2,3-bond of the acene). To explore and expand the current view, we designed and synthesized molecules where two isomeric, yet, different, 8π antiaromatic subunits, a benzocyclobutadiene (BCB) and a pentalene, are combined into, respectively, an angular and a linear topology via an unsaturated six-membered ring. The antiaromatic character of the molecules is supported experimentally by 1H NMR, UV-vis, and cyclic voltammetry measurements and X-ray crystallography. The experimental results are further confirmed by theoretical studies including the calculation of several aromaticity indices (NICS, ACID, HOMA, FLU, MCI). In the case of the angular molecule, double bond-localization within the connecting six-membered ring resulted in reduced antiaromaticity of both the BCB and pentalene subunits, while the linear structure provided a competitive situation for the two unequal [4n]π subunits. We found that in the latter case the BCB unit alleviated its unfavorable antiaromaticity more efficiently, leaving the pentalene with strong antiaromaticity. Thus, a reversed structure-antiaromaticity relationship when compared to aceno-pentalenes was achieved.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Breslow R. Antiaromaticity. Acc. Chem. Res. 1973, 6, 393–398. 10.1021/ar50072a001. - DOI
-
- Minkin V. I.; Glukhovtsev M. N.; Simkin B. Y.. Aromaticity and Antiaromaticity; John Wiley and Sons: New York, 1994.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

