Hyperbaric oxygen therapy for mild traumatic brain injury persistent postconcussion syndrome: a randomized controlled trial
- PMID: 32189664
- PMCID: PMC7871939
- DOI: 10.4103/2045-9912.279978
Hyperbaric oxygen therapy for mild traumatic brain injury persistent postconcussion syndrome: a randomized controlled trial
Abstract
Persistent postconcussion syndrome (PPCS) after mild traumatic brain injury (mTBI) is a significant public health and military problem for which there is limited treatment evidence. The aim of this study was to determine whether forty 150 kPa hyperbaric oxygen therapies (HBOTs) can improve symptoms and cognitive function in subjects with the PPCS of mTBI, using a randomized controlled crossover design with 2-month follow-up. Sixty-three civilian and military subjects with mTBI/PPCS were randomized to either 40 HBOTs at 150 kPa/60 minutes, once daily, 5 days per week in 8 weeks or an equivalent no-treatment control period. The Control Group was then crossed over to HBOT. Subjects underwent symptom, neuropsychological, and psychological testing, before and after treatment or control with retesting 2 months after the 40th HBOT. Fifty subjects completed the protocol with primary outcome testing. HBOT subjects experienced significant improvements in Neurobehavioral Symptom Inventory, Memory Index, Automated Neuropsychological Assessment Metrics, Hamilton Depression Scale, Hamilton Anxiety Scale, Post-Traumatic Stress Disorder Checklist, Pittsburgh Sleep Quality Index, and Quality Of Life after Brain Injury compared to the Control Group. After crossing over to HBOT the Control Group experienced near-identical significant improvements. Further improvements were experienced by both groups during the 2-month follow-up period. These data indicate that 40 HBOTs at 150 kPa/60 minutes demonstrated statistically significant improvements in postconcussion and Post-Traumatic Stress Disorder symptoms, memory, cognitive functions, depression, anxiety, sleep, and quality of life in civilian and military subjects with mTBI/PPCS compared to controls. Improvements persisted at least 2 months after the 40th HBOT. The study was registered on ClinicalTrials.gov (NCT02089594) on March 18, 2014 and with the U.S. Food and Drug Administration under Investigational New Drug #113823. The Institutional Review Boards of the United States Army Medical Research and Materiel Command Office of Research Protections Human Research Protection Office and the Louisiana State University School of Medicine (approval No. 7381) approved the study on May 13, 2014 and December 20, 2013, respectively.
Keywords: chronic brain injury; hyperbaric oxygen therapy; neurobehavioral symptom inventory; neuropsychological testing; neurorehabilitation; persistent postconcussion syndrome; post-traumatic stress disorder; randomized controlled trial; symptoms; traumatic brain injury.
Conflict of interest statement
None
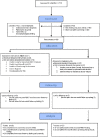
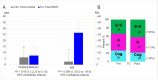
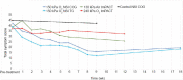
Figures

References
-
- Centers for Disease Control and Prevention. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Vol 45. Centers for Disease Control and Prevention. Atlanta, GA, USA: 2003.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

