High-Resolution Confocal Fluorescence Imaging of Serine Hydrolase Activity in Cryosections - Application to Glioma Brain Unveils Activity Hotspots Originating from Tumor-Associated Neutrophils
- PMID: 32190011
- PMCID: PMC7073015
- DOI: 10.1186/s12575-020-00118-4
High-Resolution Confocal Fluorescence Imaging of Serine Hydrolase Activity in Cryosections - Application to Glioma Brain Unveils Activity Hotspots Originating from Tumor-Associated Neutrophils
Abstract
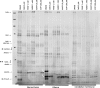
Background: Serine hydrolases (SHs) are a functionally diverse family of enzymes playing pivotal roles in health and disease and have emerged as important therapeutic targets in many clinical conditions. Activity-based protein profiling (ABPP) using fluorophosphonate (FP) probes has been a powerful chemoproteomic approach in studies unveiling roles of SHs in various biological systems. ABPP utilizes cell/tissue proteomes and features the FP-warhead, linked to a fluorescent reporter for in-gel fluorescence imaging or a biotin tag for streptavidin enrichment and LC-MS/MS-based target identification. Existing ABPP approaches characterize global SH activity based on mobility in gel or MS-based target identification and cannot reveal the identity of the cell-type responsible for an individual SH activity originating from complex proteomes.
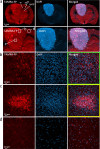
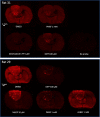
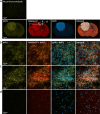
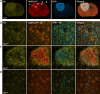
Results: Here, by using an activity probe with broad reactivity towards the SH family, we advance the ABPP methodology to glioma brain cryosections, enabling for the first time high-resolution confocal fluorescence imaging of global SH activity in the tumor microenvironment. Tumor-associated cell types were identified by extensive immunohistochemistry on activity probe-labeled sections. Tissue-ABPP indicated heightened SH activity in glioma vs. normal brain and unveiled activity hotspots originating from tumor-associated neutrophils (TANs), rather than tumor-associated macrophages (TAMs). Thorough optimization and validation was provided by parallel gel-based ABPP combined with LC-MS/MS-based target verification.
Conclusions: Our study advances the ABPP methodology to tissue sections, enabling high-resolution confocal fluorescence imaging of global SH activity in anatomically preserved complex native cellular environment. To achieve global portrait of SH activity throughout the section, a probe with broad reactivity towards the SH family members was employed. As ABPP requires no a priori knowledge of the identity of the target, we envisage no imaginable reason why the presently described approach would not work for sections regardless of species and tissue source.
Keywords: Activity-based protein profiling (ABPP); Brain cryosection; Glioblastoma multiforme (GBM); Immunohistochemistry; Neutrophil serine protease (NSP); Serine hydrolase activity; TAMRA-FP probe; Tumor-associated neutrophils.
© The Author(s) 2020.
Conflict of interest statement
Competing InterestsThe authors declare that they have no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

