Generation of Otic Lineages from Integration-Free Human-Induced Pluripotent Stem Cells Reprogrammed by mRNAs
- PMID: 32190057
- PMCID: PMC7068143
- DOI: 10.1155/2020/3692937
Generation of Otic Lineages from Integration-Free Human-Induced Pluripotent Stem Cells Reprogrammed by mRNAs
Abstract
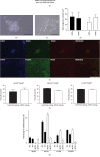
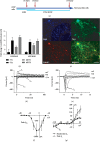
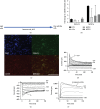
Damage to the sensory hair cells and the spiral ganglion neurons of the cochlea leads to deafness. Induced pluripotent stem cells (iPSCs) are a promising tool to regenerate the cells in the inner ear that have been affected by pathology or have been lost. To facilitate the clinical application of iPSCs, the reprogramming process should minimize the risk of introducing undesired genetic alterations while conferring the cells the capacity to differentiate into the desired cell type. Currently, reprogramming induced by synthetic mRNAs is considered to be one of the safest ways of inducing pluripotency, as the transgenes are transiently delivered into the cells without integrating into the genome. In this study, we explore the ability of integration-free human-induced pluripotent cell lines that were reprogrammed by mRNAs, to differentiate into otic progenitors and, subsequently, into hair cell and neuronal lineages. hiPSC lines were induced to differentiate by culturing them in the presence of fibroblast growth factors 3 and 10 (FGF3 and FGF10). Progenitors were identified by quantitative microscopy, based on the coexpression of otic markers PAX8, PAX2, FOXG1, and SOX2. Otic epithelial progenitors (OEPs) and otic neuroprogenitors (ONPs) were purified and allowed to differentiate further into hair cell-like cells and neurons. Lineages were characterised by immunocytochemistry and electrophysiology. Neuronal cells showed inward Na+ (I Na) currents and outward (I k) and inward K+ (I K1) currents while hair cell-like cells had inward I K1 and outward delayed rectifier K+ currents, characteristic of developing hair cells. We conclude that human-induced pluripotent cell lines that have been reprogrammed using nonintegrating mRNAs are capable to differentiate into otic cell types.
Copyright © 2020 Sarah L. Boddy et al.
Conflict of interest statement
MNR is the founder of Rinri Therapeutics.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

