Unfolded protein response (UPR) integrated signaling networks determine cell fate during hypoxia
- PMID: 32190062
- PMCID: PMC7071609
- DOI: 10.1186/s11658-020-00212-1
Unfolded protein response (UPR) integrated signaling networks determine cell fate during hypoxia
Abstract
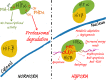
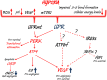
During hypoxic conditions, cells undergo critical adaptive responses that include the up-regulation of hypoxia-inducible proteins (HIFs) and the induction of the unfolded protein response (UPR). While their induced signaling pathways have many distinct targets, there are some important connections as well. Despite the extensive studies on both of these signaling pathways, the exact mechanisms involved that determine survival versus apoptosis remain largely unexplained and therefore beyond therapeutic control. Here we discuss the complex relationship between the HIF and UPR signaling pathways and the importance of understanding how these pathways differ between normal and cancer cell models.
Keywords: Angiogenesis; Cell fate determination; ER-stress; Hypoxia-reoxygenation injury; Ischemia; UPRmt.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

