Relaxation Effect of Patchouli Alcohol in Rat Corpus Cavernous and Its Underlying Mechanisms
- PMID: 32190080
- PMCID: PMC7066398
- DOI: 10.1155/2020/3109069
Relaxation Effect of Patchouli Alcohol in Rat Corpus Cavernous and Its Underlying Mechanisms
Abstract
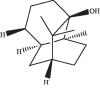
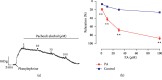
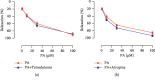
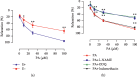
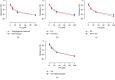
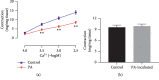
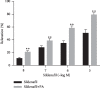
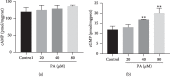
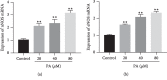
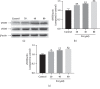
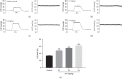
In this study, we investigated the relaxation effect and mechanisms of patchouli alcohol (PA) on rat corpus cavernosum. Corpus cavernosum strips were used in organ baths for isometric tension studies. The results showed that PA demonstrated concentration-dependent relaxation effect on rat corpus cavernosum. The relaxant response to PA was not influenced by tetrodotoxin and atropine while it was significantly inhibited by removal of endothelium. L-NG-nitroarginine methyl ester (L-NAME, a nitric oxide synthase inhibitor) or 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, a soluble guanylate cyclase inhibitor) significantly inhibited relaxation response to PA, whereas indomethacin (COX inhibitor) had no effect on PA-induced relaxation. The treatment of endothelium-deprived corpus cavernosum with several potassium channel blockers including tetraethylammonium (TEA), 4-aminopyridine (4-AP), and glibenclamide had no effect on PA-induced relaxation. Endothelium-deprived corpus cavernosal contractions induced by cumulative addition of Ca2+ to high KCl solution without CaCl2 were significantly inhibited by PA. Also, PA improved relaxant capacity of sildenafil in rat corpus cavernosum. In addition, the perfusion with PA significantly increased the levels of cGMP and expression of mRNA and protein of neuronal nitric oxide synthase (nNOS) and endothelial nitric oxide synthase (eNOS). Furthermore, intracavernous injection of PA enhanced the rise in intracavernous pressure in rats during cavernosal nerve electric stimulation. In conclusion, PA relaxed the rat corpus cavernosum attributed to both endothelium-dependent and -independent properties. While the former component was mostly involved in nitric oxide signaling pathway, the endothelium-independent mechanism involved in PA-induced relaxation was probably linked to calcium antagonism.
Copyright © 2020 Fangjun Chen et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Corbin J. D., Francis S. H. Pharmacology of phosphodiesterase-5 inhibitors. International Journal of Clinical Practice. 2002;56(6):453–459. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

