Oxidative Stress in Radiation-Induced Cardiotoxicity
- PMID: 32190171
- PMCID: PMC7071808
- DOI: 10.1155/2020/3579143
Oxidative Stress in Radiation-Induced Cardiotoxicity
Abstract
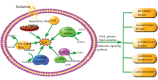
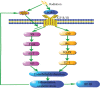
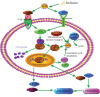
There is a distinct increase in the risk of heart disease in people exposed to ionizing radiation (IR). Radiation-induced heart disease (RIHD) is one of the adverse side effects when people are exposed to ionizing radiation. IR may come from various forms, such as diagnostic imaging, radiotherapy for cancer treatment, nuclear disasters, and accidents. However, RIHD was mainly observed after radiotherapy for chest malignant tumors, especially left breast cancer. Radiation therapy (RT) has become one of the main ways to treat all kinds of cancer, which is used to reduce the recurrence of cancer and improve the survival rate of patients. The potential cause of radiation-induced cardiotoxicity is unclear, but it may be relevant to oxidative stress. Oxidative stress, an accumulation of reactive oxygen species (ROS), disrupts intracellular homeostasis through chemical modification and damages proteins, lipids, and DNA; therefore, it results in a series of related pathophysiological changes. The purpose of this review was to summarise the studies of oxidative stress in radiotherapy-induced cardiotoxicity and provide prevention and treatment methods to reduce cardiac damage.
Copyright © 2020 Zhang Ping et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Carr Z. A., Land C. E., Kleinerman R. A., et al. Coronary heart disease after radiotherapy for peptic ulcer disease. International Journal of Radiation Oncology, Biology, Physics. 2004;61(3):842–850. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

