Trapping an unprecedented Ti3C3 unit inside the icosahedral C80 fullerene: a crystallographic survey
- PMID: 32190248
- PMCID: PMC7066662
- DOI: 10.1039/c9sc04315b
Trapping an unprecedented Ti3C3 unit inside the icosahedral C80 fullerene: a crystallographic survey
Abstract
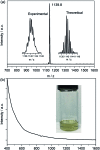
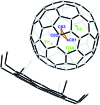
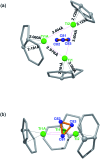
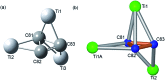
The sub-nanometer cavity of fullerene cages is an ideal platform to accommodate otherwise unstable species for accurate structural characterization with, for example, rather accurate single crystal X-ray diffraction (XRD) crystallography. Herein, we report the successful entrapment of an isolated Ti3C3 moiety inside the icosahedral-C80 cage to form Ti3C3@Ih-C80 via an arc-evaporation process in the gas phase. The single crystal XRD crystallographic results unambiguously reveal that the C3-unit adopts an unprecedented cyclopropane-like structure which coordinates with the three titanium atoms in an unexpected fashion where the triangular C3-unit is nearly perpendicular to the Ti3-plane. The intercalation of a cyclopropanated C3-unit into the titanium layer is thus unambiguously confirmed. The theoretical results reveal that the Ti3C3 cluster transfers six electrons to the Ih-C80 cage so that each titanium atom has a positive charge slightly above +2 and the C3-unit is negatively charged with about -1. It is noteworthy that this is the first observation of the cyclopropane-coordination fashion in any reported organometallic complex, providing new insights into coordination chemistry.
This journal is © The Royal Society of Chemistry 2019.
Figures
Similar articles
-
Ti3C3@C2v(9)-C82: a double-butterfly tri-metal carbide cluster inside a fullerene cage.Chem Commun (Camb). 2025 Jun 24;61(52):9432-9435. doi: 10.1039/d5cc02276b. Chem Commun (Camb). 2025. PMID: 40439569
-
Discovery of a Superatom inside the Fullerene Cage.J Phys Chem A. 2020 Apr 2;124(13):2694-2699. doi: 10.1021/acs.jpca.0c01228. Epub 2020 Mar 24. J Phys Chem A. 2020. PMID: 32167770
-
Bonding inside and outside Fullerene Cages.Acc Chem Res. 2018 Mar 20;51(3):810-815. doi: 10.1021/acs.accounts.8b00014. Epub 2018 Feb 27. Acc Chem Res. 2018. PMID: 29485263
-
Regioselective benzyl radical addition to an open-shell cluster metallofullerene. Crystallographic studies of cocrystallized Sc3C2@Ih-C80 and its singly bonded derivative.J Am Chem Soc. 2014 Jul 23;136(29):10534-40. doi: 10.1021/ja505858y. Epub 2014 Jul 14. J Am Chem Soc. 2014. PMID: 25000495
-
Endohedral Metallofullerenes: New Structures and Unseen Phenomena.Chemistry. 2020 May 7;26(26):5748-5757. doi: 10.1002/chem.201905306. Epub 2020 Mar 13. Chemistry. 2020. PMID: 31886563 Review.
Cited by
-
Recent advances in supramolecular fullerene chemistry.Chem Soc Rev. 2024 Jan 2;53(1):47-83. doi: 10.1039/d2cs00937d. Chem Soc Rev. 2024. PMID: 37853792 Free PMC article. Review.
-
Structural, Electronic, and Nonlinear Optical Properties of C66H4 and C70Cl6 Encapsulating Li and F Atoms.ACS Omega. 2021 Jun 14;6(24):16234-16240. doi: 10.1021/acsomega.1c02364. eCollection 2021 Jun 22. ACS Omega. 2021. PMID: 34179667 Free PMC article.
-
Self-driven carbon atom implantation into fullerene embedding metal-carbon cluster.Proc Natl Acad Sci U S A. 2022 Sep 27;119(39):e2202563119. doi: 10.1073/pnas.2202563119. Epub 2022 Sep 19. Proc Natl Acad Sci U S A. 2022. PMID: 36122234 Free PMC article.
-
Recent advances in endohedral metallofullerenes.Fundam Res. 2023 Dec 29;5(2):767-781. doi: 10.1016/j.fmre.2023.12.004. eCollection 2025 Mar. Fundam Res. 2023. PMID: 40242547 Free PMC article. Review.
-
Stabilizing a three-center single-electron metal-metal bond in a fullerene cage.Chem Sci. 2021 Apr 2;12(20):6890-6895. doi: 10.1039/d1sc00965f. Chem Sci. 2021. PMID: 34123317 Free PMC article.
References
-
- Lu X., Feng L., Akasaka T., Nagase S. Chem. Soc. Rev. 2012;41:7723–7760. - PubMed
-
- Bao L., Peng P., Lu X. Acc. Chem. Res. 2018;51:810–815. - PubMed
-
- Popov A. A., Yang S., Dunsch L. Chem. Rev. 2013;113:5989–6113. - PubMed
-
- Yang S., Wei T., Jin F. Chem. Soc. Rev. 2017;46:5005–5058. - PubMed
-
- Shen W., Bao L., Wu Y., Pan C., Zhao S., Fang H., Xie Y., Jin P., Peng P., Li F.-F., Lu X. J. Am. Chem. Soc. 2017;139:9979–9984. - PubMed
LinkOut - more resources
Full Text Sources

