Abortion for Fetal Genetic Abnormalities: Type of Abnormality and Gestational Age at Diagnosis
- PMID: 32190411
- PMCID: PMC7075712
- DOI: 10.1055/s-0040-1705173
Abortion for Fetal Genetic Abnormalities: Type of Abnormality and Gestational Age at Diagnosis
Abstract
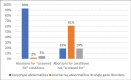
Background Advances in genetic screening can identify patients at high risk for common genetic conditions early in pregnancy and can facilitate early diagnosis and early abortion. Less common abnormalities might only be diagnosed with invasive testing is performed after structural abnormalities are identified. Objective Our objective was to compare gestational age (GA) at diagnosis and abortion for genetic abnormalities identified based on screening with abnormalities that were not discovered after screening. Study Design All prenatal diagnostic procedures from 2012 to 2017 were reviewed, and singleton pregnancies terminated following diagnosis of genetic abnormalities were identified. Cases diagnosed as the result of screening tests were compared with remaining cases. Conditions were considered "screened for" if they can be suspected by cell-free DNA testing, biochemistry, carrier screening, or if the patient was a known carrier of a single-gene disorder. When abnormal karyotype, microarray, or Noonan's syndrome was associated with abnormal NT, these cases were considered "screened for." GA at abortion was the primary outcome. Fisher's exact test and Mann-Whitney's U test were used for statistical comparison. Results In this study, 268 cases were included. A total of 227 (85%) of abortions were performed for "screened for" disorders, with 210 (93%) of these for karyotype abnormalities, 5 (2%) for microarray abnormalities, and 12 (5%) for single-gene disorders. Forty-one (15%) of abortions were performed for conditions not included in screening, with 8 (19%) of those for karyotype abnormalities, 25 (61%) for microarray abnormalities, and 8 (19%) for single-gene disorders. Invasive testing and abortion occurred at earlier median GA for those with conditions that were screened for: 12 2/7 versus 15 5/7 weeks, p ≤0.001 and 13 5/7 versus 20 0/7 weeks; p ≤0.001. Conclusion Most abortions were for abnormalities that can be suspected early in pregnancy. As many structural abnormalities associated with rare conditions are not identifiable until the mid-trimester, prenatal diagnosis and abortion occurred significantly later. Physicians and patients should be aware of the limitations of genetic screening.
Keywords: abortion; amniocentesis; chorionic villi sampling; chromosomal microarray; prenatal genetic screening; single gene disorders.
Conflict of interest statement
Conflict of Interest The authors do not have any conflict of interest.
Figures
Similar articles
-
Abortion for fetal indications: Timing of prenatal diagnosis and abortion for structural and genetic abnormalities.Contraception. 2020 May;101(5):293-295. doi: 10.1016/j.contraception.2020.02.002. Epub 2020 Feb 13. Contraception. 2020. PMID: 32061568
-
Trends in Invasive Prenatal Testing, Diagnosis, and Abortion for Fetal Aneuploidy.Am J Perinatol. 2025 Apr 30. doi: 10.1055/a-2566-9262. Online ahead of print. Am J Perinatol. 2025. PMID: 40306636
-
Clinical Practice Guidelines for Prenatal Aneuploidy Screening and Diagnostic Testing from Korean Society of Maternal-Fetal Medicine: (2) Invasive Diagnostic Testing for Fetal Chromosomal Abnormalities.J Korean Med Sci. 2021 Jan 25;36(4):e26. doi: 10.3346/jkms.2021.36.e26. J Korean Med Sci. 2021. PMID: 33496085 Free PMC article.
-
Prenatal diagnosis: choices women make about pursuing testing and acting on abnormal results.Clin Obstet Gynecol. 1993 Sep;36(3):496-509. doi: 10.1097/00003081-199309000-00008. Clin Obstet Gynecol. 1993. PMID: 8403601 Review.
-
Fetal diagnosis of inborn errors of metabolism.Baillieres Clin Obstet Gynaecol. 1987 Sep;1(3):547-67. doi: 10.1016/s0950-3552(87)80006-8. Baillieres Clin Obstet Gynaecol. 1987. PMID: 3325206 Review.
Cited by
-
Experiences of parents and stakeholders in caring for, and supporting children with special needs in Ghana.PLoS One. 2023 Mar 3;18(3):e0281502. doi: 10.1371/journal.pone.0281502. eCollection 2023. PLoS One. 2023. PMID: 36867593 Free PMC article.
-
Your baby has down syndrome: a reflexive thematic analysis of breaking the news to parents.BMC Pregnancy Childbirth. 2025 May 6;25(1):536. doi: 10.1186/s12884-025-07665-2. BMC Pregnancy Childbirth. 2025. PMID: 40329204 Free PMC article.
-
Current opportunities and new horizons into the genetic study of infertility.Rom J Morphol Embryol. 2021 Jan-Mar;62(1):191-200. doi: 10.47162/RJME.62.1.18. Rom J Morphol Embryol. 2021. PMID: 34609421 Free PMC article.
-
Stigma, Social Support, and Decision Satisfaction in Terminations of Pregnancy for Medical Reasons.Womens Health Rep (New Rochelle). 2023 May 30;4(1):271-279. doi: 10.1089/whr.2022.0092. eCollection 2023. Womens Health Rep (New Rochelle). 2023. PMID: 37284483 Free PMC article.
-
Dobbs Versus Jackson: Epilepsy, Reproductive Health, and Abortion.Epilepsy Curr. 2023 May 28;23(4):211-216. doi: 10.1177/15357597231176330. eCollection 2023 Jul-Aug. Epilepsy Curr. 2023. PMID: 37662462 Free PMC article.
References
-
- Nussbaum R L, McInnes R R, Willard H F. Philadelphia, PA: Elsevier; 2016. Principles of clinical cytogenetics and genome analysis.
-
- Practice Bulletin No. 163 Summary: screening for fetal aneuploidy. Obstet Gynecol. 2016;127(05):979–981. - PubMed
-
- et al.SMFM statement: clarification of recommendations regarding cell-free DNA aneuploidy screening. Am J Obstet Gynecol. 2015;213(06):753–754. - PubMed
-
- et al.Prenatal aneuploidy screening using cell-free DNA. Am J Obstet Gynecol. 2015;212(06):711–716. - PubMed
-
- Microarrays and next-generation sequencing technology: the use of advanced genetic diagnostic tools in obstetrics and gynecology. Obstet Gynecol. 2016;128(06):262–268. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials