Quantitative Control of Gene-Engineered T-Cell Activity through the Covalent Attachment of Targeting Ligands to a Universal Immune Receptor
- PMID: 32191035
- PMCID: PMC7306176
- DOI: 10.1021/jacs.9b11622
Quantitative Control of Gene-Engineered T-Cell Activity through the Covalent Attachment of Targeting Ligands to a Universal Immune Receptor
Abstract
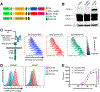
Universal immune receptors represent a rapidly emerging form of adoptive T-cell therapy with the potential to overcome safety and antigen escape challenges faced by conventional chimeric antigen receptor (CAR) T-cell therapy. By decoupling antigen recognition and T-cell signaling domains via bifunctional antigen-specific targeting ligands, universal immune receptors can regulate T-cell effector function and target multiple antigens with a single receptor. Here, we describe the development of the SpyCatcher immune receptor, the first universal immune receptor that allows for the post-translational covalent attachment of targeting ligands at the T-cell surface through the application of SpyCatcher-SpyTag chemistry. The SpyCatcher immune receptor redirected primary human T cells against a variety of tumor antigens via the addition of SpyTag-labeled targeting ligands, both in vitro and in vivo. SpyCatcher T-cell activity relied upon the presence of both target antigen and SpyTag-labeled targeting ligand, allowing for dose-dependent control of function. The mutational disruption of covalent bond formation between the receptor and the targeting ligand still permitted redirected T-cell function but significantly compromised antitumor function. Thus, the SpyCatcher immune receptor allows for rapid antigen-specific receptor assembly, multiantigen targeting, and controllable T-cell activity.
Conflict of interest statement
The authors declare the following competing financial interest(s): Daniel Powell and Andrew Tsourkas have a patent filed on this technology.
Figures

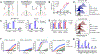


References
-
- Maude SL; Frey N; Shaw PA; Aplenc R; Barrett DM; Bunin NJ; Chew A; Gonzalez VE; Zheng Z; Lacey SF; Mahnke YD; Melenhorst JJ; Rheingold SR; Shen A; Teachey DT; Levine BL; June CH; Porter DL; Grupp SA Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med 2014, 371 (16), 1507–1517. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

