Enrollment and transition challenges in the International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) network's PROMISE trial for resource-limited regions
- PMID: 32191142
- PMCID: PMC7415523
- DOI: 10.1177/1740774520912428
Enrollment and transition challenges in the International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) network's PROMISE trial for resource-limited regions
Abstract
Background: We describe enrollment and accrual challenges in the "Promoting Maternal and Infant Survival Everywhere" (PROMISE) trial conducted in resource-limited countries, as well as the challenges in transitioning participants from the antepartum to the postpartum components of the study.
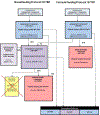
Methods: PROMISE was a large multi-national randomized controlled trial of the safety and efficacy of interventions to reduce perinatal transmission of HIV-1 (HIV) during pregnancy and breastfeeding and of interventions to preserve maternal health after cessation of perinatal transmission risk. The PROMISE study included two protocols for HIV-infected pregnant women in resource-limited countries who intended to either breastfeed or formula-feed their infants and did not meet country criteria for antiretroviral treatment. The PROMISE breastfeeding protocol (1077BF) used a sequential randomization design with up to three randomizations (Antepartum, Postpartum, and Maternal Health). The PROMISE formula-feeding protocol (1077FF) had two randomizations (Antepartum and Maternal Health). Women presenting to the clinic during early or active labor or in the immediate postpartum period were registered as Late Presenters and screened to determine whether eligible to participate in the Postpartum randomization.
Results: The study was conducted at 14 sites in seven countries and opened to enrollment in April 2011. A total of 3259 pregnant women intending to breastfeed and an additional 284 pregnant women intending to formula feed were randomized in the Antepartum component. A total of 204 Late Presenters were registered during labor or after delivery. Enrollment was high among breastfeeding women (representing 96% of the target of 3400 women) but was lower than expected among women intending to formula feed (28% of 1000 expected) and late-presenting women (8% of 2500 expected). The successful overall enrollment and final primary study analyses results were attributed to substantial preparation before the study opened, collaboration among all stakeholders, close study monitoring during implementation and the flexibility to change and streamline the protocol.
Conclusions: Experiences from the PROMISE study illustrate the challenges of enrolling in longer term studies in the setting of rapidly evolving prevention and treatment standards priorities. The lessons learned will help the community, site investigators, and study coordinators in the design and implementation of future clinical trials.
Keywords: AIDS; HIV-1; PROMISE study; antiretroviral therapy; enrollment; prevention of perinatal transmission; randomized clinical trial; resource-limited settings.
Figures
References
-
- Flynn PM, Taha TE, Cababasay M, et al. Prevention of HIV-1 transmission through breastfeeding: efficacy and safety of maternal antiretroviral therapy versus infant nevirapine prophylaxis for duration of breastfeeding in HIV-1-infected women with high CD4 cell count (IMPAACT PROMISE): a randomized, open-label, clinical trial. J Acquir Immune Defic Syndr 2018; 77: 383–392. - PMC - PubMed
-
- World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Recommendations for a public health approach, 2010. version, http://apps.who.int/iris/bitstream/10665/75236/1/9789241599818_eng.pdf (2010, accessed 30 January 2020). - PubMed
-
- WHO and UNICEF. Antenatal care in developing countries. Promises, achievements and missed opportunities. An analysis of trends, levels and differentials, 1990–2001, https://www.unicef.org/media/files/antenatal.pdf (Accessed 30 January 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

