Association of Sociodemographic and Health-Related Factors With Receipt of Nondefinitive Therapy Among Younger Men With High-Risk Prostate Cancer
- PMID: 32191331
- PMCID: PMC7082722
- DOI: 10.1001/jamanetworkopen.2020.1255
Association of Sociodemographic and Health-Related Factors With Receipt of Nondefinitive Therapy Among Younger Men With High-Risk Prostate Cancer
Erratum in
-
Error in Table 3.JAMA Netw Open. 2020 Apr 1;3(4):e205306. doi: 10.1001/jamanetworkopen.2020.5306. JAMA Netw Open. 2020. PMID: 32282042 Free PMC article. No abstract available.
Abstract
Importance: Multiple randomized clinical trials have shown that definitive therapy improves overall survival among patients with high-risk prostate cancer. However, many patients do not receive definitive therapy because of sociodemographic and health-related factors.
Objective: To identify factors associated with receipt of nondefinitive therapy (NDT) among patients aged 70 years and younger with high-risk prostate cancer.
Design, setting, and participants: This cohort study identified 72 036 patients aged 70 years and younger with high-risk prostate cancer and Charlson Comorbidity Index scores of 2 or less who were entered in the National Cancer Database between January 2004 and December 2014. Data analysis was conducted from November 2018 to December 2019.
Exposure: Receipt of NDT as an initial treatment approach.
Main outcomes and measures: Survival rates were compared based on receipt of definitive therapy or NDT, and sociodemographic and health-related factors were associated with the type of therapy received. Residual life expectancy was estimated from the National Center for Health Statistics to calculate person-years of life lost.
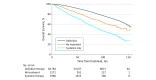
Results: A total of 72 036 men with a median (range) age of 63 (30-70) years, Charlson Comorbidity Index scores of 2 or less, and high-risk prostate cancer without regional lymph node or distant metastatic disease were analyzed. Among eligible patients, 5252 (7.3%) received NDT as an initial therapeutic strategy. On univariate and multivariate analyses, NDT was associated with worse overall survival (univariate analysis hazard ratio, 2.54; 95% CI, 2.40-2.69; P < .001; multivariate analysis hazard ratio, 2.40; 95% CI, 2.26-2.56; P < .001). Compared with patients with private insurance or managed care, those with no insurance, Medicaid, or Medicare were more likely to receive systemic therapy only (no insurance: odds ratio [OR], 3.34; 95% CI, 2.81-3.98; P < .001; Medicaid: OR, 2.92; 95% CI, 2.48-3.43; P < .001; Medicare: OR, 1.36; 95% CI, 1.20-1.53; P < .001) or no treatment (no insurance: OR, 2.63; 95% CI, 2.24-3.08; P < .001; Medicaid: OR, 1.71; 95% CI, 1.45-2.01; P < .001; Medicare: OR, 1.14; 95% CI, 1.04-1.24; P = .004). Compared with white patients, black patients were more likely to receive systemic therapy only (OR, 1.93; 95% CI, 1.74-2.14; P < .001) or no treatment (OR, 1.46; 95% CI, 1.32-1.61; P < .001), and Hispanic patients were more likely to receive systemic therapy only (OR, 1.36; 95% CI, 1.13-1.64; P = .001) or no treatment (OR, 1.36; 95% CI, 1.14-1.60; P < .001). Between 2004 and 2014, patients without insurance or enrolled in Medicaid had 1.83-fold greater person-years of life lost compared with patients with private insurance (area under the curve, 77 600 vs 42 300 person-years of life lost).
Conclusions and relevance: In this study, receipt of NDT was associated with insurance status and race/ethnicity. While treatment decisions should be individualized for every patient, younger men with high-risk prostate cancer and minimal comorbidities should be encouraged to receive definitive local therapy regardless of other factors. These data suggest that significant barriers to life-extending treatment options for patients with prostate cancer remain.
Conflict of interest statement
Figures

Similar articles
-
Racial and Ethnic Disparities in Prostate Cancer Outcomes in the Veterans Affairs Health Care System.JAMA Netw Open. 2022 Jan 4;5(1):e2144027. doi: 10.1001/jamanetworkopen.2021.44027. JAMA Netw Open. 2022. PMID: 35040965 Free PMC article.
-
Getting back to equal: The influence of insurance status on racial disparities in the treatment of African American men with high-risk prostate cancer.Urol Oncol. 2014 Nov;32(8):1285-91. doi: 10.1016/j.urolonc.2014.04.014. Epub 2014 May 17. Urol Oncol. 2014. PMID: 24846344
-
Comparison by Race of Conservative Management for Low-Risk and Intermediate-Risk Prostate Cancers in Veterans From 2004 to 2018.JAMA Netw Open. 2020 Sep 1;3(9):e2018318. doi: 10.1001/jamanetworkopen.2020.18318. JAMA Netw Open. 2020. PMID: 32986109 Free PMC article.
-
Evaluation of Social Determinants of Health and Prostate Cancer Outcomes Among Black and White Patients: A Systematic Review and Meta-analysis.JAMA Netw Open. 2023 Jan 3;6(1):e2250416. doi: 10.1001/jamanetworkopen.2022.50416. JAMA Netw Open. 2023. PMID: 36630135 Free PMC article.
-
Racial disparities in prostate cancer in the UK and the USA: similarities, differences and steps forwards.Nat Rev Urol. 2025 Apr;22(4):223-234. doi: 10.1038/s41585-024-00948-x. Epub 2024 Oct 18. Nat Rev Urol. 2025. PMID: 39424981 Review.
Cited by
-
Association of race/ethnicity and patient care experiences with receipt of definitive treatment among prostate cancer survivors: a SEER-CAHPS study.Cancer Causes Control. 2024 Apr;35(4):647-659. doi: 10.1007/s10552-023-01834-4. Epub 2023 Nov 25. Cancer Causes Control. 2024. PMID: 38001335 Free PMC article.
-
Striving for Equity: Examining Health Disparities in Urologic Oncology.Cancers (Basel). 2024 Oct 22;16(21):3559. doi: 10.3390/cancers16213559. Cancers (Basel). 2024. PMID: 39518000 Free PMC article. Review.
-
Comparing Black and White Patients in Treatment of Advanced Prostate Cancer and Survival in an Equal Access Health System.J Racial Ethn Health Disparities. 2024 Oct 21. doi: 10.1007/s40615-024-02217-4. Online ahead of print. J Racial Ethn Health Disparities. 2024. PMID: 39433656
-
Multidisciplinary clinics in prostate cancer.Oncotarget. 2021 Jul 20;12(15):1553-1554. doi: 10.18632/oncotarget.27984. eCollection 2021 Jul 20. Oncotarget. 2021. PMID: 34316333 Free PMC article. No abstract available.
-
Overcoming Disparities in Cancer: A Need for Meaningful Reform for Hispanic and Latino Cancer Survivors.Oncologist. 2021 Jun;26(6):443-452. doi: 10.1002/onco.13729. Epub 2021 Mar 10. Oncologist. 2021. PMID: 33594785 Free PMC article. Review.
References
-
- American Cancer Society Facts and figures 2019. Accessed June 15, 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-...
-
- Widmark A, Klepp O, Solberg A, et al. ; Scandinavian Prostate Cancer Group Study 7; Swedish Association for Urological Oncology 3 . Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301-308. doi:10.1016/S0140-6736(08)61815-2 - DOI - PubMed
-
- Mason MD, Parulekar WR, Sydes MR, et al. . Final report of the Intergroup Randomized Study of combined androgen-deprivation therapy plus radiotherapy versus androgen-deprivation therapy alone in locally advanced prostate cancer. J Clin Oncol. 2015;33(19):2143-2150. doi:10.1200/JCO.2014.57.7510 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

