Complement-activated interferon-γ-primed human endothelium transpresents interleukin-15 to CD8+ T cells
- PMID: 32191642
- PMCID: PMC7324183
- DOI: 10.1172/JCI135060
Complement-activated interferon-γ-primed human endothelium transpresents interleukin-15 to CD8+ T cells
Abstract
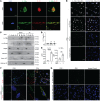
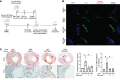
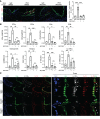
Alloantibodies in presensitized transplant candidates deposit complement membrane attack complexes (MACs) on graft endothelial cells (ECs), increasing risk of CD8+ T cell-mediated acute rejection. We recently showed that human ECs endocytose MACs into Rab5+ endosomes, creating a signaling platform that stabilizes NF-κB-inducing kinase (NIK) protein. Endosomal NIK activates both noncanonical NF-κB signaling to synthesize pro-IL-1β and an NLRP3 inflammasome to process and secrete active IL-1β. IL-1β activates ECs, increasing recruitment and activation of alloreactive effector memory CD4+ T (Tem) cells. Here, we report that IFN-γ priming induced nuclear expression of IL-15/IL-15Rα complexes in cultured human ECs and that MAC-induced IL-1β stimulated translocation of IL-15/IL-15Rα complexes to the EC surface in a canonical NF-κB-dependent process in which IL-15/IL-15Rα transpresentation increased activation and maturation of alloreactive CD8+ Tem cells. Blocking NLRP3 inflammasome assembly, IL-1 receptor, or IL-15 on ECs inhibited the augmented CD8+ Tem cell responses, indicating that this pathway is not redundant. Adoptively transferred alloantibody and mouse complement deposition induced IL-15/IL-15Rα expression by human ECs lining human coronary artery grafts in immunodeficient mice, and enhanced intimal CD8+ T cell infiltration, which was markedly reduced by inflammasome inhibition, linking alloantibody to acute rejection. Inhibiting MAC signaling may similarly limit other complement-mediated pathologies.
Keywords: Adaptive immunity; Immunology; Transplantation; endothelial cells.
Conflict of interest statement
Figures




References
-
- Biedermann BC, Pober JS. Human vascular endothelial cells favor clonal expansion of unusual alloreactive CTL. J Immunol. 1999;162(12):7022–7030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

