Prolonged inflammation leads to ongoing damage after spinal cord injury
- PMID: 32191733
- PMCID: PMC7081990
- DOI: 10.1371/journal.pone.0226584
Prolonged inflammation leads to ongoing damage after spinal cord injury
Abstract
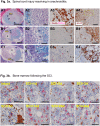
The pathogenesis of spinal cord injury (SCI) remains poorly understood and treatment remains limited. Emerging evidence indicates that post-SCI inflammation is severe but the role of reactive astrogliosis not well understood given its implication in ongoing inflammation as damaging or neuroprotective. We have completed an extensive systematic study with MRI, histopathology, proteomics and ELISA analyses designed to further define the severe protracted and damaging inflammation after SCI in a rat model. We have identified 3 distinct phases of SCI: acute (first 2 days), inflammatory (starting day 3) and resolution (>3 months) in 16 weeks follow up. Actively phagocytizing, CD68+/CD163- macrophages infiltrate myelin-rich necrotic areas converting them into cavities of injury (COI) when deep in the spinal cord. Alternatively, superficial SCI areas are infiltrated by granulomatous tissue, or arachnoiditis where glial cells are obliterated. In the COI, CD68+/CD163- macrophage numbers reach a maximum in the first 4 weeks and then decline. Myelin phagocytosis is present at 16 weeks indicating ongoing inflammatory damage. The COI and arachnoiditis are defined by a wall of progressively hypertrophied astrocytes. MR imaging indicates persistent spinal cord edema that is linked to the severity of inflammation. Microhemorrhages in the spinal cord around the lesion are eliminated, presumably by reactive astrocytes within the first week post-injury. Acutely increased levels of TNF-alpha, IL-1beta, IFN-gamma and other pro-inflammatory cytokines, chemokines and proteases decrease and anti-inflammatory cytokines increase in later phases. In this study we elucidated a number of fundamental mechanisms in pathogenesis of SCI and have demonstrated a close association between progressive astrogliosis and reduction in the severity of inflammation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010; 133: 433–447. 10.1093/brain/awp322 - DOI - PMC - PubMed
-
- Evaniew N, Noonan VK, Fallah N, Kwon BK, Rivers CS, Ahn H, et al. Methylprednisolone for the treatment of patients with acute spinal cord injuries: A propensity score-matched cohort study from a Canadian multi-center spinal cord injury registry. J Neurotrauma 32; 2015: 1674–1683. 10.1089/neu.2015.3963 - DOI - PMC - PubMed
-
- Oakden W, Kwiecien JM, O’Reilly MA, Lake E, Akens MK, Aubert I, et al. A non-invasive model of cervical spinal cord injury induced with focused ultrasound. J Neurosci Meth. 2014; 235: 92–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

