Suramin Inhibits Chikungunya Virus Replication by Interacting with Virions and Blocking the Early Steps of Infection
- PMID: 32191995
- PMCID: PMC7150963
- DOI: 10.3390/v12030314
Suramin Inhibits Chikungunya Virus Replication by Interacting with Virions and Blocking the Early Steps of Infection
Abstract
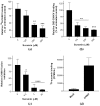
Chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus that can cause a debilitating disease that is primarily characterized by persistent joint pain. CHIKV has been emerging globally, while neither a vaccine nor antiviral medication is available. The anti-parasitic drug suramin was previously shown to inhibit CHIKV replication. In this study we aimed to obtain more detailed insight into its mechanism of action. We found that suramin interacts with virions and can inhibit virus binding to cells. It also appeared to inhibit post-attachment steps of the infection process, likely by preventing conformational changes of the envelope glycoproteins required for fusion and the progression of infection. Suramin-resistant CHIKV strains were selected and genotyping and reverse genetics experiments indicated that mutations in E2 were responsible for resistance. The substitutions N5R and H18Q were reverse engineered in the E2 glycoprotein in order to understand their role in resistance. The binding of suramin-resistant viruses with these two E2 mutations was inhibited by suramin like that of wild-type virus, but they appeared to be able to overcome the post-attachment inhibitory effect of suramin. Conversely, a virus with a G82R mutation in E2 (implicated in attenuation of vaccine strain 181/25), which renders it dependent on the interaction with heparan sulfate for entry, was more sensitive to suramin than wild-type virus. Using molecular modelling studies, we predicted the potential suramin binding sites on the mature spikes of the chikungunya virion. We conclude that suramin interferes with CHIKV entry by interacting with the E2 envelope protein, which inhibits attachment and also interferes with conformational changes required for fusion.
Keywords: CHIKV; E2 envelope protein; alphavirus; antiviral; attachment; drug repurposing; fusion; suramin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


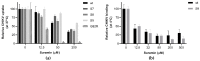

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

