Sensing Zn2+ in Aqueous Solution with a Fluorescent Scorpiand Macrocyclic Ligand Decorated with an Anthracene Bearing Tail
- PMID: 32192025
- PMCID: PMC7146481
- DOI: 10.3390/molecules25061355
Sensing Zn2+ in Aqueous Solution with a Fluorescent Scorpiand Macrocyclic Ligand Decorated with an Anthracene Bearing Tail
Abstract
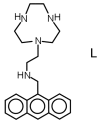
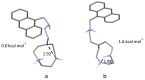
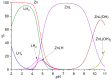
Synthesis of the new scorpiand ligand L composed of a [9]aneN3 macrocyclic ring bearing a CH2CH2NHCH2-anthracene tail is reported. L forms both cation (Zn2+) and anion (phosphate, benzoate) complexes. In addition, the zinc complexes of L bind these anions. The equilibrium constants for ligand protonation and complex formation were determined in 0.1 M NaCl aqueous solution at 298.1 ± 0.1 K by means of potentiometric (pH-metric) titrations. pH Controlled coordination/detachment of the ligand tail to Zn2+ switch on and off the fluorescence emission from the anthracene fluorophore. Accordingly, L is able to sense Zn2+ in the pH range 6-10 down to nM concentrations of the metal ion. L can efficiently sense Zn2+ even in the presence of large excess of coordinating anions, such as cyanide, sulphide, phosphate and benzoate, despite their ability to bind the metal ion.
Keywords: anion binding; azamacrocycles; chemosensor; fluorescence; scorpiand; supramolecular interactions; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Stability, water exchange, and anion binding studies on lanthanide(III) complexes with a macrocyclic ligand based on 1,7-diaza-12-crown-4: extremely fast water exchange on the Gd3+ complex.Inorg Chem. 2009 Sep 21;48(18):8878-89. doi: 10.1021/ic9011197. Inorg Chem. 2009. PMID: 19655713
-
A fluorescent chemosensor for Zn(II). Exciplex formation in solution and the solid state.Dalton Trans. 2004 Jul 21;(14):2180-7. doi: 10.1039/B403743J. Epub 2004 Jun 8. Dalton Trans. 2004. PMID: 15249955
-
A macrocyclic ligand as receptor and Zn(II)-complex receptor for anions in water: binding properties and crystal structures.Chemistry. 2011 Feb 1;17(5):1670-82. doi: 10.1002/chem.201001989. Epub 2011 Jan 5. Chemistry. 2011. PMID: 21268170
-
Syntheses, structures, and steady state and time resolved photophysical properties of a tetraiminodiphenol macrocyclic ligand and its dinuclear zinc(II)/cadmium(II) complexes with coordinating and noncoordinating anions.Inorg Chem. 2012 Aug 20;51(16):8739-49. doi: 10.1021/ic300412u. Epub 2012 Aug 7. Inorg Chem. 2012. PMID: 22871203
-
[Supramolecular chemistry of multinuclear zinc(II) complexes in aqueous solution].Yakugaku Zasshi. 2002 Dec;122(12):1095-108. doi: 10.1248/yakushi.122.1095. Yakugaku Zasshi. 2002. PMID: 12510387 Review. Japanese.
Cited by
-
Advances in the synthesis and applications of macrocyclic polyamines.R Soc Open Sci. 2024 Jun 19;11(6):231979. doi: 10.1098/rsos.231979. eCollection 2024 Jun. R Soc Open Sci. 2024. PMID: 39092147 Free PMC article. Review.
-
Pyrene-Containing Polyamines as Fluorescent Receptors for Recognition of PFOA in Aqueous Media.Molecules. 2023 Jun 5;28(11):4552. doi: 10.3390/molecules28114552. Molecules. 2023. PMID: 37299033 Free PMC article.
-
Assembly of Polyiodide Networks with Cu(II) Complexes of Pyridinol-Based Tetraaza Macrocycles.Inorg Chem. 2022 Jan 10;61(1):368-383. doi: 10.1021/acs.inorgchem.1c02967. Epub 2021 Dec 21. Inorg Chem. 2022. PMID: 34933551 Free PMC article.
-
Fluorescent molecular probe for in vivo and in vitro targeting and imaging of an intracellular bacterial infection.Chem Sci. 2025 Mar 24;16(18):7902-7911. doi: 10.1039/d4sc05680a. eCollection 2025 May 7. Chem Sci. 2025. PMID: 40191126 Free PMC article.
-
Design, Synthesis, and Biological Evaluation of Boron-Containing Macrocyclic Polyamines and Their Zinc(II) Complexes for Boron Neutron Capture Therapy.J Med Chem. 2021 Jun 24;64(12):8523-8544. doi: 10.1021/acs.jmedchem.1c00445. Epub 2021 Jun 2. J Med Chem. 2021. PMID: 34077212 Free PMC article.
References
-
- Pallavicini P.S., Perotti A., Poggi A., Seghi B., Fabbrizzi L. N-(aminoethyl)cyclam: A tetraaza macrocycle with a coordinating tail (scorpiand). Acidity controlled coordination of the side chain to nickel(II) and nickel(III) cations. J. Am. Chem. Soc. 1987;109:5139–5144. doi: 10.1021/ja00251a016. - DOI
-
- Mewis R.E., Archibald S.J. Biomedical applications of macrocyclic ligand complexes. Coord. Chem. Rev. 2010;254:1686–1712. doi: 10.1016/j.ccr.2010.02.025. - DOI
-
- Lejault P., Duskova K., Bernhard C., Valverde I.E., Romieu A., Monchaud D. The Scope of Application of Macrocyclic Polyamines beyond Metal Chelation. Eur. J. Org. Chem. 2019;2019:6146–6157. doi: 10.1002/ejoc.201900870. - DOI
-
- Tsitovich P.B., Morrow J.R. Macrocyclic ligands for Fe(II) paraCEST and chemical shift MRI contrast agents. Inorg. Chim. Acta. 2012;393:3–11. doi: 10.1016/j.ica.2012.06.010. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

