In Vitro Anti-Prostate Cancer Activity of Two Ebselen Analogues
- PMID: 32192052
- PMCID: PMC7151718
- DOI: 10.3390/ph13030047
In Vitro Anti-Prostate Cancer Activity of Two Ebselen Analogues
Abstract
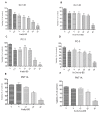
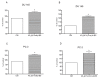
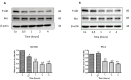
Scientific research has been underway for decades in order to develop an effective anticancer drug, and it has become crucial to find a novel and effective chemotherapeutics in the case of prostate cancer treatment. Ebselen derivatives have been shown to possess a variety of biological activities, including cytostatic and cytotoxic action against tumor cells. In this study, the cytotoxic effect and anticancer mechanism of action of two organoselenium compounds- (N-allyl-1,2-benzisoselenazol-3(2H)-one (N-allyl-BS) and N-(3-methylbutyl)-1,2-benzisoselenazol-3(2H)-one) (N-(3-mb)-BS)-were investigated on two phenotypically different prostate cancer cell lines DU 145 and PC-3. The influence of analyzed compounds on the viability parameter was also assessed on normal prostate cell line PNT1A. The results showed that both organoselenium compounds (OSCs) efficiently inhibited cancer cell proliferation, whereas normal PNT1A cells were less sensitive to the analazyed ebselen analouges. Both OSCs induced G2/M cell cycle arrest and prompted cell death through apoptosis. The detection of cleaved Poly (ADP-ribose) Polymerase (PARP) confirmed this. In addition, N-allyl-BS and N-(3-m)-b-BS increased the level of reactive oxygen species (ROS) formation, however only N-allyl-BS induced DNA damage. Based on our data, we assume that OSCs' anticancer action can be associated with oxidative stress induction and inactivation of the Akt- dependent signalling pathway. In conclusion, our data demonstrate that ebselen derivatives showed strong cytotoxic efficiency towards prostate cancer cells and may be elucidated as a novel, potent anticancer agent.
Keywords: DNA damage 4; Organoselenium compound 1; ROS 3; apoptosis 2; chemoprevention 5.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures








References
-
- Venkateswaran V., Klotz L.H., Fleshner N.E. Selenium modulation of cell proliferation and cell cycle biomarkers in human prostate carcinoma cell lines. Cancer Res. 2002;62:2540–2545. - PubMed
-
- Cho S.D., Jiang C., Malewicz B., Dong Y., Young C.Y., Kang K.S., Lee Y.S., Ip C., Lu J. Methyl selenium metabolites decrease prostate-specific antigen expression by inducing protein degradation and suppressing androgen-stimulated transcription. Mol. Cancer Ther. 2004;3:605–611. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

