Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ-ESI-MS
- PMID: 32192063
- PMCID: PMC7139372
- DOI: 10.3390/ijms21062045
Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ-ESI-MS
Abstract
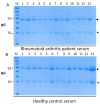
Aberrant glycosylation has been observed in many autoimmune diseases. For example, aberrant glycosylation of immunoglobulin G (IgG) has been implicated in rheumatoid arthritis (RA) pathogenesis. The aim of this study is to investigate IgG glycosylation and whether there is an association with rheumatoid factor levels in the serum of RA patients. We detected permethylated N-glycans of the IgG obtained in serum from 44 RA patients and 30 healthy controls using linear ion-trap electrospray ionization mass spectrometry (LTQ-ESI-MS), a highly sensitive and efficient approach in the detection and identification of N-glycans profiles. IgG N-glycosylation and rheumatoid factor levels were compared in healthy controls and RA patients. Our results suggested that total IgG purified from serum of RA patients shows significantly lower galactosylation (p = 0.0012), lower sialylation (p < 0.0001) and higher fucosylation (p = 0.0063) levels compared with healthy controls. We observed a positive correlation between aberrant N-glycosylation and rheumatoid factor level in the RA patients. In conclusion, we identified aberrant glycosylation of IgG in the serum of RA patients and its association with elevated levels of rheumatoid factor.
Keywords: IgG; glycosylation; mass spectrometry; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


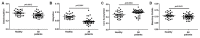

Similar articles
-
Characterization of IgG glycosylation in rheumatoid arthritis patients by MALDI-TOF-MSn and capillary electrophoresis.Anal Bioanal Chem. 2017 Jun;409(15):3731-3739. doi: 10.1007/s00216-017-0302-1. Epub 2017 Apr 10. Anal Bioanal Chem. 2017. PMID: 28397166
-
Hypogalactosylation of immunoglobulin G in rheumatoid arthritis: relationship to HLA-DRB1 shared epitope, anticitrullinated protein antibodies, rheumatoid factor, and correlation with inflammatory activity.Arthritis Res Ther. 2018 Mar 14;20(1):44. doi: 10.1186/s13075-018-1540-0. Arthritis Res Ther. 2018. PMID: 29540200 Free PMC article.
-
Possible role of β-galactosidase in rheumatoid arthritis.Mod Rheumatol. 2020 Jul;30(4):671-680. doi: 10.1080/14397595.2019.1640175. Epub 2019 Jul 22. Mod Rheumatol. 2020. PMID: 31269834
-
[Defect of glycosylation of immunoglobulin G in rheumatoid arthritis patients].Postepy Hig Med Dosw (Online). 2005;59:485-9. Postepy Hig Med Dosw (Online). 2005. PMID: 16258413 Review. Polish.
-
[The diagnostic value of IgG galactosylation in rheumatoid arthritis].Orv Hetil. 1997 Jun 15;138(24):1577-8. Orv Hetil. 1997. PMID: 9254375 Review. Hungarian.
Cited by
-
Changes of serum IgG glycosylation patterns in rheumatoid arthritis.Clin Proteomics. 2023 Feb 21;20(1):7. doi: 10.1186/s12014-023-09395-z. Clin Proteomics. 2023. PMID: 36810000 Free PMC article.
-
Novel Biomarkers for Diagnosis and Monitoring of Immune Thrombocytopenia.Int J Mol Sci. 2023 Feb 23;24(5):4438. doi: 10.3390/ijms24054438. Int J Mol Sci. 2023. PMID: 36901864 Free PMC article. Review.
-
Physiological and Pathological Inflammation Induced by Antibodies and Pentraxins.Cells. 2021 May 12;10(5):1175. doi: 10.3390/cells10051175. Cells. 2021. PMID: 34065953 Free PMC article. Review.
-
Medical Relevance, State-of-the-Art and Perspectives of "Sweet Metacode" in Liquid Biopsy Approaches.Diagnostics (Basel). 2024 Mar 28;14(7):713. doi: 10.3390/diagnostics14070713. Diagnostics (Basel). 2024. PMID: 38611626 Free PMC article. Review.
-
Quantitative N-glycoproteomic analysis reveals glycosylation signatures of plasma immunoglobulin G in systemic sclerosis.Front Immunol. 2025 Feb 7;16:1531191. doi: 10.3389/fimmu.2025.1531191. eCollection 2025. Front Immunol. 2025. PMID: 39991159 Free PMC article.
References
-
- Parekh R.B., Dwek R.A., Sutton B.J., Fernandes D.L., Leung A., Stanworth D., Rademacher T.W., Mizuochi T., Taniguchi T., Matsuta K., et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–457. doi: 10.1038/316452a0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

