Effect of Streptozotocin-Inducted Diabetes on the Pathophysiology of Enteric Neurons in the Small Intestine Based on the Porcine Diabetes Model
- PMID: 32192078
- PMCID: PMC7139978
- DOI: 10.3390/ijms21062047
Effect of Streptozotocin-Inducted Diabetes on the Pathophysiology of Enteric Neurons in the Small Intestine Based on the Porcine Diabetes Model
Abstract
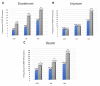
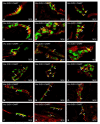
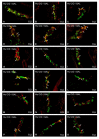
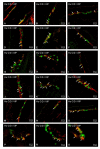
Hyperglycemia is one of the main causes of diabetes complications. Gastrointestinal (GI) disturbances are one of the most frequent complications during diabetes. The porcine digestive tract possesses physiological and pathological similarities to the human digestive tract. This also applies to the innervation of the gastrointestinal tract. In this study, the influence of experimentally-inducted hyperglycemia was examined on the expression of vesicular acetylcholine transporter (VAChT), cocaine- and amphetamine-regulated transcript (CART), galanin (GAL), vasoactive intestinal polypeptide (VIP), and calcitonin gene-related peptide (CGRP) in the enteric nervous system (ENS) neurons in the small intestine of the pig. During the current study, an increased number of neurons containing CART, VIP, GAL, and CGRP under streptozotocin injection were observed. The augmentation of expression included all enteric plexuses present in the small intestine. The same results were obtained in the case of VAChT; namely, chronic hyperglycemia led to an increase in the number of neurons utilizing VAChT in all investigated plexuses. The obtained results suggested that the function of neuropeptides studied in this experiment depended on their localization in the ENS structures, as well as part of the GI tract. Diabetes led to alterations in the neurochemical phenotype of small intestine enteric neurons.
Keywords: diabetes; enteric nervous system; gastrointestinal complications; neuropeptides; pig.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



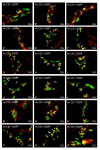
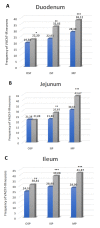
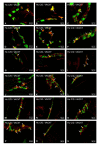
References
-
- Furness J.B., Callaghan B.P., Rivera L.R., Cho H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014;817:39–71. - PubMed
-
- Furness J.B. The enteric nervous system: Normal functions and enteric neuropathies. Neurogastroenterol. Motil. 2008;20(Suppl. 1):32–38. - PubMed
-
- Schemann M., Neunlist M. The human enteric nervous system. Neurogastroenterol. Motil. 2004;16(Suppl. 1):55–59. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

