Microbiome Profile of Deep Endometriosis Patients: Comparison of Vaginal Fluid, Endometrium and Lesion
- PMID: 32192080
- PMCID: PMC7151170
- DOI: 10.3390/diagnostics10030163
Microbiome Profile of Deep Endometriosis Patients: Comparison of Vaginal Fluid, Endometrium and Lesion
Abstract
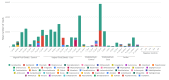
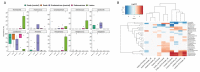
This work aimed to identify and compare the bacterial patterns present in endometriotic lesions, eutopic endometrium and vaginal fluid from endometriosis patients with those found in the vaginal fluid and eutopic endometrium of control patients. Vaginal fluid, eutopic endometrium and endometriotic lesions were collected. DNA was extracted and the samples were analyzed to identify microbiome by high-throughput DNA sequencing of the 16S rRNA marker gene. Amplicon sequencing from vaginal fluid, eutopic endometrium and endometriotic lesion resulted in similar profiles of microorganisms, composed most abundantly by the genus Lactobacillus, Gardnerella, Streptococcus and Prevotella. No significant differences were found in the diversity analysis of microbiome profiles between control and endometriotic patients; however deep endometriotic lesions seems to present different bacterial composition, less predominant of Lactobacillus and with more abundant Alishewanella, Enterococcus and Pseudomonas.
Keywords: 16S rRNA; endometriosis; microbiome; next generation sequencing (NGS); pathogenesis; vaginal fluid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources

