Gut-Liver Axis and Inflammasome Activation in Cholangiocyte Pathophysiology
- PMID: 32192118
- PMCID: PMC7140657
- DOI: 10.3390/cells9030736
Gut-Liver Axis and Inflammasome Activation in Cholangiocyte Pathophysiology
Abstract
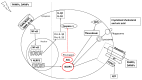
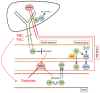
The Nlrp3 inflammasome is a multiprotein complex activated by a number of bacterial products or danger signals and is involved in the regulation of inflammatory processes through caspase-1 activation. The Nlrp3 is expressed in immune cells but also in hepatocytes and cholangiocytes, where it appears to be involved in regulation of biliary damage, epithelial barrier integrity and development of fibrosis. Activation of the pathways of innate immunity is crucial in the pathophysiology of hepatobiliary diseases, given the strong link between the gut and the liver. The liver secretes bile acids, which influence the bacterial composition of the gut microbiota and, in turn, are heavily modified by microbial metabolism. Alterations of this balance, as for the development of dysbiosis, may deeply influence the composition of the bacterial products that reach the liver and are able to activate a number of intracellular pathways. This alteration may be particularly important in the pathogenesis of cholangiopathies and, in particular, of primary sclerosing cholangitis, given its strong association with inflammatory bowel disease. In the present review, we summarize current knowledge on the gut-liver axis in cholangiopathies and discuss the role of Nlrp3 inflammasome activation in cholestatic conditions.
Keywords: NLRP3; cholangiocyte; gut–liver axis; inflammasome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis.Gut. 2019 Aug;68(8):1477-1492. doi: 10.1136/gutjnl-2018-316670. Epub 2019 Mar 14. Gut. 2019. PMID: 30872395
-
Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis.Oncotarget. 2016 Dec 20;7(51):83951-83963. doi: 10.18632/oncotarget.13796. Oncotarget. 2016. PMID: 27924062 Free PMC article.
-
Pathophysiologic implications of innate immunity and autoinflammation in the biliary epithelium.Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt B):1374-1379. doi: 10.1016/j.bbadis.2017.07.023. Epub 2017 Jul 25. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 28754453 Free PMC article. Review.
-
Roles of the inflammasome in the gut‑liver axis (Review).Mol Med Rep. 2019 Jan;19(1):3-14. doi: 10.3892/mmr.2018.9679. Epub 2018 Nov 20. Mol Med Rep. 2019. PMID: 30483776 Free PMC article. Review.
-
Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases.Autoimmun Rev. 2017 Sep;16(9):885-896. doi: 10.1016/j.autrev.2017.07.002. Epub 2017 Jul 8. Autoimmun Rev. 2017. PMID: 28698093 Review.
Cited by
-
NLRP3 Inflammasome in Acute and Chronic Liver Diseases.Int J Mol Sci. 2024 Apr 20;25(8):4537. doi: 10.3390/ijms25084537. Int J Mol Sci. 2024. PMID: 38674122 Free PMC article. Review.
-
Stimulator of IFN genes mediates neuroinflammatory injury by suppressing AMPK signal in experimental subarachnoid hemorrhage.J Neuroinflammation. 2020 May 25;17(1):165. doi: 10.1186/s12974-020-01830-4. J Neuroinflammation. 2020. PMID: 32450897 Free PMC article.
-
Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation.Acta Pharmacol Sin. 2021 Feb;42(2):230-241. doi: 10.1038/s41401-020-0490-7. Epub 2020 Aug 7. Acta Pharmacol Sin. 2021. PMID: 32770173 Free PMC article.
-
New insights into the interplay between intestinal flora and bile acids in inflammatory bowel disease.World J Clin Cases. 2022 Oct 26;10(30):10823-10839. doi: 10.12998/wjcc.v10.i30.10823. World J Clin Cases. 2022. PMID: 36338232 Free PMC article. Review.
-
Purinergic Signaling in Non-Parenchymal Liver Cells.Int J Mol Sci. 2024 Aug 30;25(17):9447. doi: 10.3390/ijms25179447. Int J Mol Sci. 2024. PMID: 39273394 Free PMC article. Review.
References
-
- Hiramatsu K., Harada K., Tsuneyama K., Sasaki M., Fujita S., Hashimoto T., Kaneko S., Kobayashi K., Nakanuma Y. Amplification and sequence analysis of partial bacterial 16S ribosomal RNA gene in gallbladder bile from patients with primary biliary cirrhosis. J. Hepatol. 2000;33:9–18. doi: 10.1016/S0168-8278(00)80153-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

