Induction of Heat Shock Protein 70 in Mouse RPE as an In Vivo Model of Transpupillary Thermal Stimulation
- PMID: 32192227
- PMCID: PMC7139698
- DOI: 10.3390/ijms21062063
Induction of Heat Shock Protein 70 in Mouse RPE as an In Vivo Model of Transpupillary Thermal Stimulation
Abstract
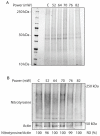
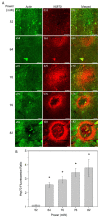
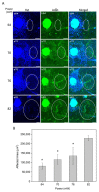
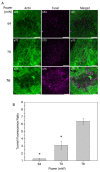
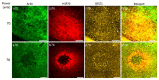
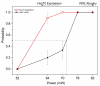
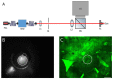
The induction of heat shock response in the macula has been proposed as a useful therapeutic strategy for retinal neurodegenerative diseases by promoting proteostasis and enhancing protective chaperone mechanisms. We applied transpupillary 1064 nm long-duration laser heating to the mouse (C57Bl/6J) fundus to examine the heat shock response in vivo. The intensity and spatial distribution of heat shock protein (HSP) 70 expression along with the concomitant probability for damage were measured 24 h after laser irradiation in the mouse retinal pigment epithelium (RPE) as a function of laser power. Our results show that the range of heating powers for producing heat shock response while avoiding damage in the mouse RPE is narrow. At powers of 64 and 70 mW, HSP70 immunostaining indicates 90 and 100% probability for clearly elevated HSP expression while the corresponding probability for damage is 20 and 33%, respectively. Tunel staining identified the apoptotic regions, and the estimated 50% damaging threshold probability for the heating (ED50) was ~72 mW. The staining with Bestrophin1 (BEST1) demonstrated RPE cell atrophy with the most intense powers. Consequently, fundus heating with a long-duration laser provides an approachable method to develop heat shock-based therapies for the RPE of retinal disease model mice.
Keywords: age-related macular degeneration (AMD); heat shock protein 70 (HSP70); immunohistology; mouse; retinal pigment epithelium (RPE); transpupillary laser-induced heating.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Expression of heat shock protein 70 and cell death kinetics after different thermal impacts on cultured retinal pigment epithelial cells.Exp Eye Res. 2018 May;170:117-126. doi: 10.1016/j.exer.2018.02.013. Epub 2018 Feb 16. Exp Eye Res. 2018. PMID: 29454858
-
Non-damaging retinal phototherapy: dynamic range of heat shock protein expression.Invest Ophthalmol Vis Sci. 2011 Mar 28;52(3):1780-7. doi: 10.1167/iovs.10-5917. Invest Ophthalmol Vis Sci. 2011. PMID: 21087969
-
Transpupillary thermotherapy for age-related macular degeneration: long-pulse photocoagulation, apoptosis, and heat shock proteins.Ophthalmic Surg Lasers. 2000 Sep-Oct;31(5):359-73. Ophthalmic Surg Lasers. 2000. PMID: 11011704 Review.
-
Protective effect of a laser-induced sub-lethal temperature rise on RPE cells from oxidative stress.Exp Eye Res. 2014 Jul;124:37-47. doi: 10.1016/j.exer.2014.04.014. Epub 2014 May 5. Exp Eye Res. 2014. PMID: 24800654
-
Heat shock proteins as gatekeepers of proteolytic pathways-Implications for age-related macular degeneration (AMD).Ageing Res Rev. 2009 Apr;8(2):128-39. doi: 10.1016/j.arr.2009.01.001. Ageing Res Rev. 2009. PMID: 19274853 Review.
Cited by
-
Hormetic Heat Shock Enhances Autophagy through HSF1 in Retinal Pigment Epithelium Cells.Cells. 2022 May 28;11(11):1778. doi: 10.3390/cells11111778. Cells. 2022. PMID: 35681472 Free PMC article.
-
Phototherapy techniques for the management of musculoskeletal disorders: strategies and recent advances.Biomater Res. 2023 Nov 28;27(1):123. doi: 10.1186/s40824-023-00458-8. Biomater Res. 2023. PMID: 38017585 Free PMC article. Review.
-
An imbalance in autophagy contributes to retinal damage in a rat model of oxygen-induced retinopathy.J Cell Mol Med. 2021 Nov;25(22):10480-10493. doi: 10.1111/jcmm.16977. Epub 2021 Oct 8. J Cell Mol Med. 2021. PMID: 34623024 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

