Transcription factors and transporters in zinc homeostasis: lessons learned from fungi
- PMID: 32192376
- PMCID: PMC7770983
- DOI: 10.1080/10409238.2020.1742092
Transcription factors and transporters in zinc homeostasis: lessons learned from fungi
Abstract
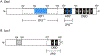
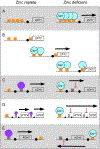
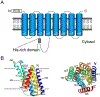
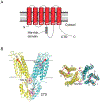
Zinc is an essential nutrient for all organisms because this metal serves as a critical structural or catalytic cofactor for many proteins. These zinc-dependent proteins are abundant in the cytosol as well as within organelles of eukaryotic cells such as the nucleus, mitochondria, endoplasmic reticulum, Golgi, and storage compartments such as the fungal vacuole. Therefore, cells need zinc transporters so that they can efficiently take up the metal and move it around within cells. In addition, because zinc levels in the environment can vary drastically, the activity of many of these transporters and other components of zinc homeostasis is regulated at the level of transcription by zinc-responsive transcription factors. Mechanisms of post-transcriptional control are also important for zinc homeostasis. In this review, the focus will be on our current knowledge of zinc transporters and their regulation by zinc-responsive transcription factors and other mechanisms in fungi because these organisms have served as useful paradigms of zinc homeostasis in all organisms. With this foundation, extension to other organisms will be made where warranted.
Keywords: Zinc; homeostasis; regulation; transcription factors; transporters.
Conflict of interest statement
DECLARATION OF INTEREST STATEMENT
The author has no conflict of interest to declare related to this work. Work in the author’s lab was supported by the National Institute of Health, primarily by grant R01-GM56285.
Figures

References
-
- Amich J, Leal F, Calera JA. (2009).Repression of the acid ZrfA/ZrfB zinc-uptake system of Aspergillus fumigatus mediated by PacC under neutral, zinc-limiting conditions. Int Microbiol, 12, 39–47 - PubMed
-
- Amich J, Vicentefranqueira R, Mellado E, Ruiz-Carmuega A, Leal F, Calera JA. (2014).The ZrfC alkaline zinc transporter is required for Aspergillus fumigatus virulence and its growth in the presence of the Zn/Mn-chelating protein calprotectin. Cell Microbiol, 16, 548–64 - PubMed
-
- Andreini C, Banci L, Bertini I, Rosato A. (2006).Zinc through the three domains of life. J Proteome Res, 5, 3173–8 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
