Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury
- PMID: 32192523
- PMCID: PMC7082961
- DOI: 10.1186/s12974-020-01761-0
Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury
Abstract
Background: The interaction between astrocytes and microglia plays a vital role in the damage and repair of brain lesions due to traumatic brain injury (TBI). Recent studies have shown that exosomes act as potent mediators involved in intercellular communication.
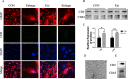
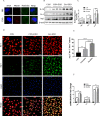
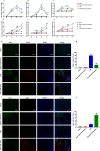
Methods: In the current study, the expression of inflammatory factors and miR-873a-5p in the lesion area and oedema area was evaluated in 15 patients with traumatic brain injury. Exosomes secreted by astrocytes were detected by immunofluorescence, Western blot and electron microscopy. A mouse model of TBI and an in vitro model of LPS-induced primary microglia were established to study the protective mechanism of exosomes from miR-873a-5p overexpressing in TBI-induced nerve injury.
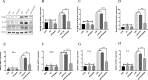
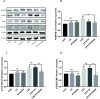
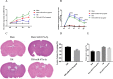
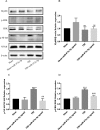
Results: We discovered that exosomes derived from activated astrocytes promote microglial M2 phenotype transformation following TBI. More than 100 miRNAs were detected in these astrocyte-derived exosomes. miR-873a-5p is a major component that was highly expressed in human traumatic brain tissue. Moreover, miR-873a-5p significantly inhibited LPS-induced microglial M1 phenotype transformation and the subsequent inflammation through decreased phosphorylation of ERK and NF-κB p65. This effect also greatly improved the modified neurological severity score (mNSS) and attenuated brain injury in a strictly controlled cortical impact mouse model.
Conclusions: Taken together, our research indicates that miRNAs in the exosomes derived from activated astrocytes play a key role in the astrocyte-microglia interaction. miR-873a-5p, as one of the main components of these astrocyte-derived exosomes, attenuated microglia-mediated neuroinflammation and improved neurological deficits following TBI by inhibiting the NF-κB signalling pathway. These findings suggest a potential role for miR-873a-5p in treating traumatic brain injury.
Keywords: Astrocyte; Exosome; M1/M2; Microglia; Traumatic brain injury; miR-873a-5p.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

