Risk of colorectal cancer incidence and mortality after polypectomy: a Swedish record-linkage study
- PMID: 32192628
- PMCID: PMC7234902
- DOI: 10.1016/S2468-1253(20)30009-1
Risk of colorectal cancer incidence and mortality after polypectomy: a Swedish record-linkage study
Abstract
Background: Long-term colorectal cancer incidence and mortality after colorectal polyp removal remains unclear. We aimed to assess colorectal cancer incidence and mortality in individuals with removal of different histological subtypes of polyps relative to the general population.
Methods: We did a matched cohort study through prospective record linkage in Sweden in patients aged at least 18 years with a first diagnosis of colorectal polyps in the nationwide gastrointestinal ESPRESSO histopathology cohort (1993-2016). For each polyp case, we identified up to five matched reference individuals from the Total Population Register on the basis of birth year, age, sex, calendar year of biopsy, and county of residence. We excluded patients and reference individuals with a diagnosis of colorectal cancer either before or within the first 6 months after diagnosis of the index polyp. Polyps were classified by morphology codes into hyperplastic polyps, sessile serrated polyps, tubular adenomas, tubulovillous adenomas, and villous adenomas. Colorectal cancer cases were identified from the Swedish Cancer Registry, and cause-of-death data were retrieved from the Cause of Death Register. We collected information about the use of endoscopic examination before and after the index biopsy from the Swedish National Patient Registry, and counted the number of endoscopies done before and after the index biopsies. We calculated cumulative risk of colorectal cancer incidence and mortality at 3, 5, 10, and 15 years, and computed hazard ratios (HRs) and 95% CIs for colorectal cancer incidence and mortality using a stratified Cox proportional hazards model within each of the matched pairs.
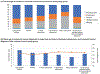
Findings: 178 377 patients with colorectal polyps and 864 831 matched reference individuals from the general population were included in our study. The mean age of patients at polyp diagnosis was 58·6 (SD 13·9) years for hyperplastic polyps, 59·7 (14·2) years for sessile serrated polyps, 63·9 (12·9) years for tubular adenomas, 67·1 (12·1) years for tubulovillous adenomas, and 68·9 (11·8) years for villous adenomas. During a median of 6·6 years (IQR 3·0-11·6) of follow-up, we documented 4278 incident colorectal cancers and 1269 colorectal cancer-related deaths in patients with a polyp, and 14 350 incident colorectal cancers and 5242 colorectal cancer deaths in general reference individuals. The 10-year cumulative incidence of colorectal cancer was 1·6% (95% CI 1·5-1·7) for hyperplastic polyps, 2·5% (1·9-3·3) for sessile serrated polyps, 2·7% (2·5-2·9) for tubular adenomas, 5·1% (4·8-5·4) for tubulovillous adenomas, and 8·6% (7·4-10·1) for villous adenomas compared with 2·1% (2·0-2·1) in reference individuals. Compared with reference individuals, patients with any polyps had an increased risk of colorectal cancer, with multivariable HR of 1·11 (95% CI 1·02-1·22) for hyperplastic polyps, 1·77 (1·34-2·34) for sessile serrated polyps, 1·41 (1·30-1·52) for tubular adenomas, 2·56 (2·36-2·78) for tubulovillous adenomas, and 3·82 (3·07-4·76) for villous adenomas (p<0·05 for all polyp subtypes). There was a higher proportion of incident proximal colon cancer in patients with serrated (hyperplastic and sessile) polyps (52-57%) than in those with conventional (tubular, tubulovillous, and villous) adenomas (30-46%). For colorectal cancer mortality, a positive association was found for sessile serrated polyps (HR 1·74, 95% CI 1·08-2·79), tubulovillous adenomas (1·95, 1·69-2·24), and villous adenomas (3·45, 2·40-4·95), but not for hyperplastic polyps (0·90, 0·76-1·06) or tubular adenomas (0·97, 0·84-1·12).
Interpretation: In a largely screening-naive population, compared with individuals from the general population, patients with any polyps had a higher colorectal cancer incidence, and those with sessile serrated polyps, tubulovillous adenomas, and villous adenomas had a higher colorectal cancer mortality.
Funding: US National Institutes of Health, American Cancer Society, American Gastroenterological Association, Union for International Cancer Control.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures












Comment in
-
Sessile serrated polyps and colorectal cancer mortality.Lancet Gastroenterol Hepatol. 2020 Jun;5(6):516-517. doi: 10.1016/S2468-1253(20)30074-1. Epub 2020 Mar 17. Lancet Gastroenterol Hepatol. 2020. PMID: 32192629 No abstract available.
References
-
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138(6): 2088–100. - PubMed
-
- JE IJ, Vermeulen L, Meijer GA, Dekker E. Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol 2015; 12(7): 401–9. - PubMed
-
- Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010; 59(5): 666–89. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

