C-FGF23 peptide alleviates hypoferremia during acute inflammation
- PMID: 32193252
- PMCID: PMC7849576
- DOI: 10.3324/haematol.2019.237040
C-FGF23 peptide alleviates hypoferremia during acute inflammation
Abstract
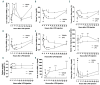
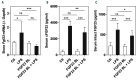
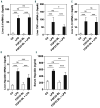
Hypoferremia results as an acute phase response to infection and inflammation aiming to reduce iron availability to pathogens. Activation of toll-like receptors (TLRs), the key sensors of the innate immune system, induces hypoferremia mainly through the rise of the iron hormone hepcidin. Conversely, stimulation of erythropoiesis suppresses hepcidin expression via induction of the erythropoietin-responsive hormone erythroferrone. Iron deficiency stimulates transcription of the osteocyte-secreted protein FGF23. Here we hypothesized that induction of FGF23 in response to TLR4 activation is a potent contributor to hypoferremia and, thus, impairment of its activity may alleviate hypoferremia induced by lipopolysaccharide (LPS), a TLR 4 agonist. We used the C-terminal tail of FGF23 to impair endogenous full-length FGF23 signaling in wild-type mice, and investigated its impact on hypoferremia. Our data show that FGF23 is induced as early as pro-inflammatory cytokines in response to LPS, followed by upregulation of hepcidin and downregulation of erythropoietin (Epo) expression in addition to decreased serum iron and transferrin saturation. Further, LPS-induced hepatic and circulating hepcidin were significantly reduced by FGF23 signaling disruption. Accordingly, iron sequestration in liver and spleen caused by TLR4 activation was completely abrogated by FGF23 signaling inhibition, resulting in alleviation of serum iron and transferrin saturation deficit. Taken together, our studies highlight for the first time that inhibition of FGF23 signaling alleviates LPS-induced acute hypoferremia.
Figures




Comment in
-
Ironing out an approach to alleviate the hypoferremia of acute inflammation.Haematologica. 2021 Feb 1;106(2):326-328. doi: 10.3324/haematol.2020.266627. Haematologica. 2021. PMID: 33522782 Free PMC article. No abstract available.
References
-
- Layoun A, Huang H, Calve A, Santos MM. Toll-like receptor signal adaptor protein MyD88 is required for sustained endotoxin- induced acute hypoferremic response in mice. Am J Pathol. 2012;180(6):2340-2350. - PubMed
-
- Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783-788. - PubMed
-
- Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106(5):1864-1866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

