Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer
- PMID: 32193378
- PMCID: PMC7081234
- DOI: 10.1038/s41467-020-15315-8
Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer
Erratum in
-
Author Correction: Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer.Nat Commun. 2020 May 18;11(1):2543. doi: 10.1038/s41467-020-16344-z. Nat Commun. 2020. PMID: 32424117 Free PMC article.
Abstract
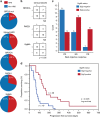
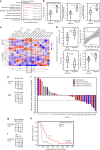
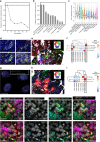
Combined PARP and immune checkpoint inhibition has yielded encouraging results in ovarian cancer, but predictive biomarkers are lacking. We performed immunogenomic profiling and highly multiplexed single-cell imaging on tumor samples from patients enrolled in a Phase I/II trial of niraparib and pembrolizumab in ovarian cancer (NCT02657889). We identify two determinants of response; mutational signature 3 reflecting defective homologous recombination DNA repair, and positive immune score as a surrogate of interferon-primed exhausted CD8 + T-cells in the tumor microenvironment. Presence of one or both features associates with an improved outcome while concurrent absence yields no responses. Single-cell spatial analysis reveals prominent interactions of exhausted CD8 + T-cells and PD-L1 + macrophages and PD-L1 + tumor cells as mechanistic determinants of response. Furthermore, spatial analysis of two extreme responders shows differential clustering of exhausted CD8 + T-cells with PD-L1 + macrophages in the first, and exhausted CD8 + T-cells with cancer cells harboring genomic PD-L1 and PD-L2 amplification in the second.
Conflict of interest statement
J.G. and Y.Z. were previously employed by TESARO, and now is a GSK employee. B.D. was an employee of Tesaro at the time of the work related to this manuscript. G.I.S. receives research funding from Merck & Co. S.S. is a consultant for RareCyte, Inc. P.K.S. is a member of the SAB or Board of Directors of Applied Biomath and RareCyte Inc and has equity in these companies. In the last 5 years, the Sorger lab has received research funding from Novartis and Merck. Sorger declares that none of these relationships are directly or indirectly related to the content of this manuscript. For the SigMA algorithm, a patent application titled “Systems and methods for classifying tumors” has been filed under the Patent Cooperation Treaty (PCT) by authors D.G. and P.P. on 18 September, 2019 following a provisional patent application filed on 24 September, 2018. U.M. has served as a consultant for Merck and has been the North America PI of the NOVA study funded by TESARO. P.K. and A.D.D’A. have served as consultants/members of advisory boards for Merck and Tesaro/GSK. Other authors have no competing interests to declare.
Figures


References
-
- Matulonis, U. A. et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase 2 KEYNOTE-100 study. Ann Oncol. 30, 1080–1087 (2019). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

