The role of semen and seminal plasma in inducing large-scale genomic changes in the female porcine peri-ovulatory tract
- PMID: 32193402
- PMCID: PMC7081221
- DOI: 10.1038/s41598-020-60810-z
The role of semen and seminal plasma in inducing large-scale genomic changes in the female porcine peri-ovulatory tract
Abstract
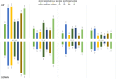
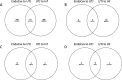
Semen modifies the expression of genes related to immune function along the porcine female internal genital tract. Whether other pathways are induced by the deposition of spermatozoa and/or seminal plasma (SP), is yet undocumented. Here, to determine their relative impact on the uterine and tubal transcriptomes, microarray analyses were performed on the endocervix, endometrium and endosalpinx collected from pre-ovulatory sows 24 h after either mating or artificial insemination (AI) with specific ejaculate fractions containing spermatozoa or sperm-free SP. After enrichment analysis, we found an overrepresentation of genes and pathways associated with sperm transport and binding, oxidative stress and cell-to-cell recognition, such as PI3K-Akt, FoxO signaling, glycosaminoglycan biosynthesis and cAMP-related transcripts, among others. Although semen (either after mating or AI) seemed to have the highest impact along the entire genital tract, our results demonstrate that the SP itself also modifies the transcriptome. The detected modifications of the molecular profiles of the pre/peri-ovulatory endometrium and endosalpinx suggest an interplay for the survival, transport and binding of spermatozoa through, for instance the up-regulation of the Estrogen signaling pathway associated with attachment and release from the oviductal reservoir.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Langendijk P, Soede NM, Kemp B. Uterine activity, sperm transport, and the role of boar stimuli around insemination in sows. Theriogenology. 2005;63:500–513. - PubMed
-
- Georgiou AS, et al. Modulation of the oviductal environment by gametes. J. Proteome Res. 2007;6:4656–4666. - PubMed
-
- Maillo V, et al. Oviductal response to gametes and early embryos in mammals. Reproduction. 2016;152:R127–41. - PubMed
-
- Rodriguez-Martinez H, et al. The physiological roles of the boar ejaculate. Soc. Reprod. Fertil. Suppl. 2009;66:1–21. - PubMed

