In-line filtration in very preterm neonates: a randomized controlled trial
- PMID: 32193413
- PMCID: PMC7081338
- DOI: 10.1038/s41598-020-61815-4
In-line filtration in very preterm neonates: a randomized controlled trial
Abstract
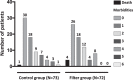
In-line filtration is increasingly used in critically-ill infants but its benefits, by preventing micro-particle infusion in very preterm neonates, remain to be demonstrated. We conducted a randomized controlled trial among very preterm infants allocated to receive either in-line filtration of all the intra-venous lines or standard care without filters. The primary outcome was differences greater than 20% in the median changes in pro-inflammatory cytokine serum concentrations measured at day 3 and day 8 (+/-1) using a Luminex multianalytic profiling technique. Major neonatal complications were analyzed as secondary predefined outcomes. We randomized 146 infants, assigned to filter (n = 73) or control (n = 73) group. Difference over 20% in pro-inflammatory cytokine concentration between day 3 and day 8 was not found statistically different between the two groups, both in intent-to-treat (with imputation) and per protocol (without imputation) analyses. The incidences of most of neonatal complications were found to be similar. Hence, this trial did not evidence a beneficial effect of in-line filtration in very preterm infants on the inflammatory response syndrome and neonatal morbidities. These data should be interpreted according to local standards in infusion preparation and central line management.
Conflict of interest statement
Andreas Capewell is an employee of Pall Medical, SLS, Dreieich, Germany. The other authors have no conflict of interest to disclose.
Figures
References
-
- Kuban KCK, et al. Association of Circulating Proinflammatory and Anti-inflammatory Protein Biomarkers in Extremely Preterm Born Children with Subsequent Brain Magnetic Resonance Imaging Volumes and Cognitive Function at Age 10 Years. J. Pediatr. 2019;210:81–90 e83. doi: 10.1016/j.jpeds.2019.03.018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical