Nuclear receptor CAR-ERα signaling regulates the estrogen sulfotransferase gene in the liver
- PMID: 32193417
- PMCID: PMC7081254
- DOI: 10.1038/s41598-020-61767-9
Nuclear receptor CAR-ERα signaling regulates the estrogen sulfotransferase gene in the liver
Abstract
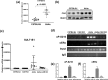
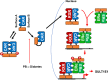
Estrogen sulfotransferase (SULT1E1) inactivates estrogen and regulates its metabolic homeostats. Whereas SULT1E1 is expressed low in the liver of adult mice, it is induced by phenobarbital (PB) treatment or spontaneously in diabetic livers via nuclear receptors. Utilizing constitutive active/androstane receptor (CAR) KO, estrogen receptor α (ERα KO, phosphorylation-blocked ERα S216A KI mice, it is now demonstrated that, after being activated by PB, CAR binds and recruits ERα onto the Sulte1 promoter for subsequent phosphorylation at Ser216. This phosphorylation tightens CAR interacting with ERα and to activates the promoter. Hepatic SULT1E1 mRNA levels are constitutively up-regulated in type 1 diabetic Akita mice; CAR spontaneously accumulates in the nucleus and activates the Sult1e1 promoter by recruiting phosphorylated ERα in the liver as observed with PB-induced livers. Thus, this CAR-phosphorylated ERα signaling enables these two nuclear receptors to communicate, activating the Sult1e1 gene in response to either PB or diabetes in mice. ERα phosphorylation may integrate CAR into estrogen actions, providing insights into understanding drug-hormone interactions in clinical therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Phenobarbital-induced phosphorylation converts nuclear receptor RORα from a repressor to an activator of the estrogen sulfotransferase gene Sult1e1 in mouse livers.FEBS Lett. 2018 Aug;592(16):2760-2768. doi: 10.1002/1873-3468.13199. Epub 2018 Aug 10. FEBS Lett. 2018. PMID: 30025153 Free PMC article.
-
Immunoprecipitation Analyses of Estrogen Receptor α Phosphorylated at Serine 216 in the Mouse Liver.Methods Mol Biol. 2022;2418:41-51. doi: 10.1007/978-1-0716-1920-9_3. Methods Mol Biol. 2022. PMID: 35119658
-
PXR phosphorylated at Ser350 transduces a glucose signal to repress the estrogen sulfotransferase gene in human liver cells and fasting signal in mouse livers.Biochem Pharmacol. 2020 Oct;180:114197. doi: 10.1016/j.bcp.2020.114197. Epub 2020 Aug 14. Biochem Pharmacol. 2020. PMID: 32798464
-
Estrogen sulfotransferase in the metabolism of estrogenic drugs and in the pathogenesis of diseases.Expert Opin Drug Metab Toxicol. 2019 Apr;15(4):329-339. doi: 10.1080/17425255.2019.1588884. Epub 2019 Mar 18. Expert Opin Drug Metab Toxicol. 2019. PMID: 30822161 Free PMC article.
-
Regulation of gene expression by CAR: an update.Arch Toxicol. 2015 Jul;89(7):1045-55. doi: 10.1007/s00204-015-1522-9. Epub 2015 May 16. Arch Toxicol. 2015. PMID: 25975989 Review.
Cited by
-
Phenobarbital in Nuclear Receptor Activation: An Update.Drug Metab Dispos. 2023 Feb;51(2):210-218. doi: 10.1124/dmd.122.000859. Epub 2022 Nov 9. Drug Metab Dispos. 2023. PMID: 36351837 Free PMC article. Review.
-
Candidate Proficiency Test Chemicals to Address Industrial Chemical Applicability Domains for in vitro Human Cytochrome P450 Enzyme Induction.Front Toxicol. 2022 Jun 20;4:880818. doi: 10.3389/ftox.2022.880818. eCollection 2022. Front Toxicol. 2022. PMID: 35795225 Free PMC article. Review.
-
Hepatic Gene Expression and Metabolite Profiles of Androstenone and Skatole Relative to Plasma Estrone Sulfate Levels in Boars.Biomolecules. 2024 Jul 15;14(7):850. doi: 10.3390/biom14070850. Biomolecules. 2024. PMID: 39062564 Free PMC article.
-
Sex-specific expression mechanism of hepatic estrogen inactivating enzyme and transporters in diabetic women.Biochem Pharmacol. 2021 Aug;190:114662. doi: 10.1016/j.bcp.2021.114662. Epub 2021 Jun 23. Biochem Pharmacol. 2021. PMID: 34157297 Free PMC article.
-
RORα phosphorylation by casein kinase 1α as glucose signal to regulate estrogen sulfation in human liver cells.Biochem J. 2020 Sep 30;477(18):3583-3598. doi: 10.1042/BCJ20200427. Biochem J. 2020. PMID: 32686824 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

