Migratory dendritic cells in skin-draining lymph nodes have nickel-binding capabilities
- PMID: 32193426
- PMCID: PMC7081353
- DOI: 10.1038/s41598-020-61875-6
Migratory dendritic cells in skin-draining lymph nodes have nickel-binding capabilities
Abstract
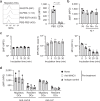
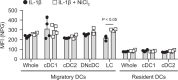
Nickel (Ni) is the most frequent metal allergen and induces Th1-dependent type-IV allergies. In local skin, epidermal Langerhans cells (LCs) and/or dermal dendritic cells (DCs) uptake antigens and migrate to draining lymph nodes (LNs). However, the subsets of antigen-presenting cells that contribute to Ni presentation have not yet been identified. In this study, we analyzed the Ni-binding capabilities of murine DCs using fluorescent metal indicator Newport Green. Elicitation of Ni allergy was assessed after intradermal (i.d.) injection of Ni-treated DCs into ear pinnae of Ni-sensitized mice. The Ni-binding capabilities of MHC class IIhi CD11cint migratory DCs were significantly stronger than those of MHC class IIint CD11chi resident DCs and CD11cint PDCA1+ MHC class IIint B220+ plasmacytoid DCs. Migratory DCs in skin-draining and mandibular LNs showed significantly stronger Ni-binding capabilities than those in mesenteric and medial iliac LNs. An i.d. injection of IL-1β induced the activation of LCs and dermal DCs with strong Ni-binding capabilities. Ni-binding LCs were detected in draining LNs after i.d. challenge with IL-1β and Ni. Moreover, an i.d. injection of Ni-treated DCs purified from skin-draining LNs elicited Ni-allergic inflammation. These results demonstrated that migratory DCs in skin-draining LNs have strong Ni-binding capabilities and elicit Ni allergy.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

