Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at multi-locations in Texas
- PMID: 32193449
- PMCID: PMC7081193
- DOI: 10.1038/s41598-020-61696-7
Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at multi-locations in Texas
Abstract
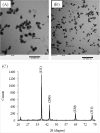
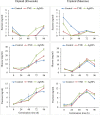
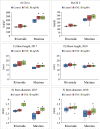
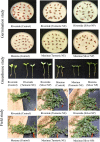
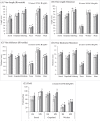
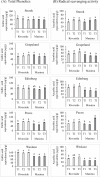
Seed priming uses treatments to improve seed germination and thus potentially increase growth and yield. Low-cost, environmentally friendly, effective seed treatment remain to be optimized and tested for high-value specialty crop like watermelon (Citrullus lanatus) in multi-locations. This remains a particularly acute problem for triploids, which produce desirable seedless watermelons, but show low germination rates. In the present study, turmeric oil nanoemulsions (TNE) and silver nanoparticles (AgNPs) synthesized from agro-industrial byproducts were used as nanopriming agents for diploid (Riverside) and triploid (Maxima) watermelon seeds. Internalization of nanomaterials was confirmed by neutron activation analysis, transmission electron microscopy, and gas chromatography-mass spectrometry. The seedling emergence rate at 14 days after sowing was significantly higher in AgNP-treated triploid seeds compared to other treatments. Soluble sugar (glucose and fructose) contents were enhanced during germination in the AgNP-treated seeds at 96 h. Seedlings grown in the greenhouse were transplanted at four locations in Texas: Edinburg, Pecos, Grapeland, and Snook in 2017. At Snook, higher yield 31.6% and 35.6% compared to control were observed in AgNP-treated Riverside and Maxima watermelons, respectively. To validate the first-year results, treated and untreated seeds of both cultivars were sown in Weslaco, Texas in 2018. While seed emegence and stand establishments were enhanced by seed priming, total phenolics radical-scavenging activities, and macro- and microelements in the watermelon fruits were not significantly different from the control. The results of the present study demonstracted that seed priming with AgNPs can enhance seed germination, growth, and yield while maintaining fruit quality through an eco-friendly and sustainable nanotechnological approach.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Chen K, Arora R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013;94:33–45. doi: 10.1016/j.envexpbot.2012.03.005. - DOI
-
- Maynard, D. An introduction to the watermelon. (ASHS Press, Alexandria, 2001).
-
- Wang, T., Cobb, B., Sittertz-Bhatkar, H. & Leskovar, D. in XXVI International Horticultural Congress: Issues and Advances in Transplant Production and Stand Establishment Research 631. 71–77.
-
- Wang T, Sistrunk L, Leskovar D, Cobb B. Characteristics of storage reserves of triploid watermelon seeds: association of starch and mean germination time. Seed Sci. Technol. 2011;39:318–326. doi: 10.15258/sst.2011.39.2.05. - DOI
-
- Grange S, Leskovar DI, Pike LM, Cobb BG. Seedcoat structure and oxygen-enhanced environments affect germination of triploid watermelon. J. Am. Soc. Hortic. Sci. 2003;128:253–259. doi: 10.21273/JASHS.128.2.0253. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

