Benchmarking Observational Analyses Against Randomized Trials: a Review of Studies Assessing Propensity Score Methods
- PMID: 32193818
- PMCID: PMC7210373
- DOI: 10.1007/s11606-020-05713-5
Benchmarking Observational Analyses Against Randomized Trials: a Review of Studies Assessing Propensity Score Methods
Abstract
Background: Observational analysis methods can be refined by benchmarking against randomized trials. We reviewed studies systematically comparing observational analyses using propensity score methods against randomized trials to explore whether intervention or outcome characteristics predict agreement between designs.
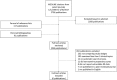
Methods: We searched PubMed (from January 1, 2000, to April 30, 2017), the AHRQ Scientific Resource Center Methods Library, reference lists, and bibliographies to identify systematic reviews that compared estimates from observational analyses using propensity scores against randomized trials across three or more clinical topics; reported extractable relative risk (RR) data; and were published in English. One reviewer extracted data from all eligible systematic reviews; a second reviewer verified the extracted data.
Results: Six systematic reviews matching published observational studies to randomized trials, published between 2012 and 2016, met our inclusion criteria. The reviews reported on 127 comparisons overall, in cardiology (29 comparisons), surgery (49), critical care medicine and sepsis (46), nephrology (2), and oncology (1). Disagreements were large (relative RR < 0.7 or > 1.43) in 68 (54%) and statistically significant in 12 (9%) of the comparisons. The degree of agreement varied among reviews but was not strongly associated with intervention or outcome characteristics.
Discussion: Disagreements between observational studies using propensity score methods and randomized trials can occur for many reasons and the available data cannot be used to discern the reasons behind specific disagreements. Better benchmarking of observational analyses using propensity scores (and other causal inference methods) is possible using observational studies that explicitly attempt to emulate target trials.
Keywords: benchmarking; comparative effectiveness; observational studies; propensity score; randomized controlled trials.
Conflict of interest statement
The authors declare that they do not have a conflict of interest.
Figures

References
-
- Fisher RA. The design of experiments. London: Oliver And Boyd; Edinburgh; 1937.
-
- Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Boston: Houghton Mifflin; 2001.
-
- Dahabreh IJ. Randomization, randomized trials, and analyses using observational data: A commentary on Deaton and Cartwright. Soc Sci Med. 2018;210:41–44. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

