Use of Magnetic Resonance Neurography for Evaluating the Distribution and Patterns of Chronic Inflammatory Demyelinating Polyneuropathy
- PMID: 32193896
- PMCID: PMC7082655
- DOI: 10.3348/kjr.2019.0739
Use of Magnetic Resonance Neurography for Evaluating the Distribution and Patterns of Chronic Inflammatory Demyelinating Polyneuropathy
Abstract
Objective: To evaluate the distribution and characteristics of peripheral nerve abnormalities in chronic inflammatory demyelinating polyneuropathy (CIDP) using magnetic resonance neurography (MRN) and to examine the diagnostic efficiency.
Materials and methods: Thirty-one CIDP patients and 21 controls underwent MR scans. Three-dimensional sampling perfections with application-optimized contrasts using different flip-angle evolutions and T1-/T2- weighted turbo spin-echo sequences were performed for neurography of the brachial and lumbosacral (LS) plexus and cauda equina, respectively. Clinical data and scores of the inflammatory Rasch-built overall disability scale (I-RODS) in CIDP were obtained.
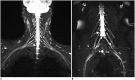
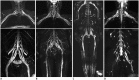
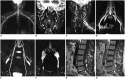
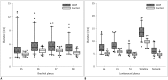
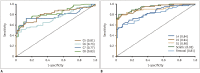
Results: The bilateral extracranial vagus (n = 11), trigeminal (n = 12), and intercostal nerves (n = 10) were hypertrophic. Plexus hypertrophies were observed in the brachial plexus of 19 patients (61.3%) and in the LS plexus of 25 patients (80.6%). Patterns of hypertrophy included uniform hypertrophy (17 [54.8%] brachial plexuses and 21 [67.7%] LS plexuses), and multifocal fusiform hypertrophy (2 [6.5%] brachial plexuses and 4 [12.9%] LS plexuses) was present. Enlarged and/or contrast-enhanced cauda equina was found in 3 (9.7%) and 13 (41.9%) patients, respectively. Diameters of the brachial and LS nerve roots were significantly larger in CIDP than in controls (p < 0.001). The largest AUC was obtained for the L5 nerve. There were no significant differences in the course duration, I-RODS score, or diameter between patients with and without hypertrophy.
Conclusion: MRN is useful for the assessment of distribution and characteristics of the peripheral nerves in CIDP. Compared to other regions, LS plexus neurography is more sensitive for CIDP.
Keywords: Brachial plexus; Chronic inflammatory demyelinating polyneuropathy; Cranial nerves; Lumbosacral plexus; Magnetic resonance neurography.
Copyright © 2020 The Korean Society of Radiology.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
References
-
- Vallat JM, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. Lancet Neurol. 2010;9:402–412. - PubMed
-
- Rotta FT, Sussman AT, Bradley WG, Ram Ayyar D, Sharma KR, Shebert RT. The spectrum of chronic inflammatory demyelinating polyneuropathy. J Neurol Sci. 2000;173:129–139. - PubMed
-
- Alabdali M, Abraham A, Alsulaiman A, Breiner A, Barnett C, Katzberg HD, et al. Clinical characteristics, and impairment and disability scale scores for different CIDP disease activity status classes. J Neurol Sci. 2017;372:223–227. - PubMed
-
- de Silva RN, Willison HJ, Doyle D, Weir AI, Hadley DM, Thomas AM. Nerve root hypertrophy in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 1994;17:168–170. - PubMed
-
- Iijima M, Koike H, Hattori N, Tamakoshi A, Katsuno M, Tanaka F, et al. Prevalence and incidence rates of chronic inflammatory demyelinating polyneuropathy in the Japanese population. J Neurol Neurosurg Psychiatry. 2008;79:1040–1043. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

