Genetic Hearing Loss Associated With Autoinflammation
- PMID: 32194497
- PMCID: PMC7066252
- DOI: 10.3389/fneur.2020.00141
Genetic Hearing Loss Associated With Autoinflammation
Abstract
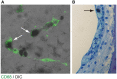
Sensorineural hearing loss can result from dysfunction of the inner ear, auditory nerve, or auditory pathways in the central nervous system. Sensorineural hearing loss can be associated with age, exposure to ototoxic drugs or noise, or mutations in nuclear or mitochondrial genes. However, it is idiopathic in some patients. Although these disorders are mainly caused by dysfunction of the inner ear, little of the pathophysiology in sensorineural hearing loss is known due to inaccessibility of the living human inner ear for biopsy and pathological analysis. The inner ear has previously been thought of as an immune-privileged organ. We recently showed that a missense mutation of the NLRP3 gene is associated with autosomal-dominant sensorineural hearing loss with cochlear autoinflammation in two unrelated families. NLRP3 encodes the NLRP3 protein, a key component of the NLRP3 inflammasome that is expressed in immune cells, including monocytes and macrophages. Gain-of-function mutations of NLRP3 cause abnormal activation of the NLRP3 inflammasome leading to IL-1β secretion in a spectrum of autosomal dominant systemic autoinflammatory phenotypes termed cryopyrin-associated periodic syndromes. The affected subjects of our two families demonstrated atypical phenotypes compared with those reported for subjects with cryopyrin-associated periodic syndromes. These observations led us to test the hypothesis that macrophage/monocyte-like cells in the cochlea can mediate local autoinflammation via activation of the NLRP3 inflammasome. The inflammasome can indeed be activated in macrophage/monocyte-like cells of the mouse cochlea, with secretion of IL-1β. The macrophage/monocyte-like cells in the cochlea were also found to be associated with hearing loss in a Slc26a4-insufficient mouse model of human deafness. This review addresses our understanding of genetic hearing loss mediated by autoinflammation and macrophage/monocyte-like cells in the cochlea.
Keywords: NLRP3; Pendred syndrome; SLC26A4; cryopyrin-associated periodic syndromes; hearing loss; interleukin-1β; macrophage.
Copyright © 2020 Nakanishi, Prakash, Ito, Kim, Brewer, Harrow, Roux, Hosokawa and Griffith.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

