Adoptive Cell Therapy Targeting Neoantigens: A Frontier for Cancer Research
- PMID: 32194541
- PMCID: PMC7066210
- DOI: 10.3389/fimmu.2020.00176
Adoptive Cell Therapy Targeting Neoantigens: A Frontier for Cancer Research
Abstract
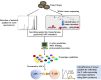
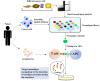
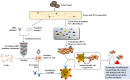
Adoptive cell therapy (ACT) is a kind of immunotherapy in which T cells are genetically modified to express a chimeric antigen receptor (CAR) or T cell receptor (TCR), and ACT has made a great difference in treating multiple types of tumors. ACT is not perfect, and it can be followed by severe side effects, which hampers the application of ACT in clinical trials. One of the most promising methods to minimize side effects is to endow adoptive T cells with the ability to target neoantigens, which are specific to tumor cells. With the development of antigen screening technologies, more methods can be applied to discover neoantigens in cancer cells, such as whole-exome sequencing combined with mass spectrometry, neoantigen screening through an inventory-shared neoantigen peptide library, and neoantigen discovery via trogocytosis. In this review, we focus on the side effects of existing antigens and their solutions, illustrate the strategies of finding neoantigens in CAR-T and TCR-T therapies through methods reported by other researchers, and summarize the clinical behavior of these neoantigens.
Keywords: CAR-T; TCR-T; adoptive cell therapy; neoantigen; neoantigen screening.
Copyright © 2020 Wang and Cao.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

