Gender Disparity Impacts on Thymus Aging and LHRH Receptor Antagonist-Induced Thymic Reconstitution Following Chemotherapeutic Damage
- PMID: 32194555
- PMCID: PMC7062683
- DOI: 10.3389/fimmu.2020.00302
Gender Disparity Impacts on Thymus Aging and LHRH Receptor Antagonist-Induced Thymic Reconstitution Following Chemotherapeutic Damage
Abstract
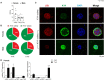
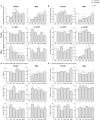
One of the main consequences of thymus aging is the decrease in naïve T cell output. This condition accelerates at the onset of puberty, and presents as a major clinical complication for cancer patients who require cytoablative therapy. Specifically, the extensive use of chemotherapeutics, such as cyclophosphamide, in such treatments damage thymic structure and eliminate the existing naïve T cell repertoire. The resulting immunodeficiency can lead to increased incidence of opportunistic infections, tumor growth relapse and/or autoimmune diseases, particularly in older patients. Thus, strategies aimed at rejuvenating the aged thymus following chemotherapeutic damage are required. Previous studies have revealed that sex hormone deprivation in male mice is capable of regenerating the thymic microenvironment following chemotherapy treatment, however, further investigation is crucial to identify gender-based differences, and the molecular mechanisms involved during thymus regeneration. Through phenotypic analyzes, we identified gender-specific alterations in thymocytes and thymic epithelial cell (TEC) subsets from the onset of puberty. By middle-age, females presented with a higher number of thymocytes in comparison to males, yet a decrease in their Aire+ medullary TEC/thymocyte ratio was observed. This reduction could be associated with an increased risk of autoimmune disease in middle-aged women. Given the concurrent increase in female Aire+ cTEC/thymocyte ratio, we proposed that there may be an impediment in Aire+ mTEChi differentiation, and Aire+ cTEChi as its upstream precursor. The regenerative effects of LHRH receptor antagonist, degarelix, on TEC subsets was also less pronounced in middle-aged females compared to males, possibly due to slower progression of thymic involution in the former, which presented with greater TEChi proportions. Furthermore, following cyclophosphamide treatment, degarelix enhanced thymocyte and mature TEC subset recovery, with faster recovery kinetics observed in females. These events were found to involve both reactivation and proliferation of thymic epithelial progenitor cells. Taken together, the findings from this study portray a relationship between gender disparity and thymus aging, and highlight the potential benefits of LHRH receptor antagonist treatment for thymic regeneration. Further research is required, however, to determine how gender may impact on the mechanisms underpinning these events.
Keywords: aging; chemotherapy; gender; luteinizing hormone-releasing hormone; regeneration; sex hormone deprivation; thymic epithelial cell; thymus.
Copyright © 2020 Hun, Wong, Gunawan, Alsharif, Quinn and Chidgey.
Figures






References
-
- Mackall C, Fleisher T, Brown M, Magrath I, Shad A, Horowitz M, et al. . Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood. (1994) 84:2221–8. - PubMed
-
- Popa I, Zubkova I, Medvedovic M, Romantseva T, Mostowski H, Boyd R, et al. . Regeneration of the adult thymus is preceded by the expansion of K5+ K8+ epithelial cell progenitors and by increased expression of Trp63, cMyc and Tcf3 transcription factors in the thymic stroma. Int Immunol. (2007) 19:1249–60. 10.1093/intimm/dxm092 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

