Liposomal Delivery of the RNA Genome of a Live-Attenuated Chikungunya Virus Vaccine Candidate Provides Local, but Not Systemic Protection After One Dose
- PMID: 32194557
- PMCID: PMC7066069
- DOI: 10.3389/fimmu.2020.00304
Liposomal Delivery of the RNA Genome of a Live-Attenuated Chikungunya Virus Vaccine Candidate Provides Local, but Not Systemic Protection After One Dose
Abstract
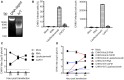
Chikungunya virus (CHIKV) is the causative pathogen of chikungunya fever, a mosquito-borne viral disease causing highly debilitating arthralgia that can persist for months and progress to chronic arthritis. Our previous studies have identified the CHIKV live-attenuated vaccine candidate CHIKV-NoLS. Like most live-attenuated vaccines, attenuated replication of CHIKV-NoLS has the potential to limit scalable production. To overcome production limits, as well as other drawbacks of live-attenuated vaccines, we developed an in vivo liposome RNA delivery system to deliver the self-replicating RNA genome of CHIKV-NoLS directly into mice, allowing the recipients' body to produce the live-attenuated vaccine particles. CAF01 liposomes were able to deliver replication-competent CHIKV-NoLS RNA in vitro. Immunodeficient AG129 mice inoculated with liposome-delivered CHIKV-NoLS RNA developed viremia and disease signs representative of this lethal model of CHIKV infection, demonstrating de novo vaccine particle production in vivo. In immunocompetent C57BL/6 mice, liposome-delivered CHIKV-NoLS RNA inoculation was associated with reduced IgM and IgG levels with low antibody CHIKV-neutralizing capacity, compared to vaccination with the original live-attenuated vaccine CHIKV-NoLS. One dose of liposome-delivered CHIKV-NoLS RNA did not provide systemic protection from CHIKV wild-type (WT) challenge but was found to promote an early onset of severe CHIKV-induced footpad swelling. Liposome-delivered CHIKV-NoLS RNA inoculation did, however, provide local protection from CHIKV-WT challenge in the ipsilateral foot after one dose. Results suggest that in the presence of low CHIKV-specific neutralizing antibody levels, local inflammatory responses, likely brought on by liposome adjuvants, have a role in the protection of CHIKV-induced footpad swelling in the ipsilateral foot of mice inoculated with liposome-delivered CHIKV-NoLS RNA. Low IgG and CHIKV-specific neutralizing antibody levels may be responsible for early onset of severe swelling in the feet of CHIKV-WT-challenged mice. These results support previous studies that suggest CHIKV is vulnerable to antibody-mediated enhancement of disease. Further studies using booster regimes aim to demonstrate the potential for liposomes to deliver the self-replicating RNA genome of live-attenuated vaccines and offer a novel immunization strategy.
Keywords: RNA; chikungunya; liposome; live-attenuated; vaccine.
Copyright © 2020 Abeyratne, Tharmarajah, Freitas, Mostafavi, Mahalingam, Zaid, Zaman and Taylor.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

