Oligopeptide-modified poly(beta-amino ester)s-coated AdNuPARmE1A: Boosting the efficacy of intravenously administered therapeutic adenoviruses
- PMID: 32194832
- PMCID: PMC7052890
- DOI: 10.7150/thno.40902
Oligopeptide-modified poly(beta-amino ester)s-coated AdNuPARmE1A: Boosting the efficacy of intravenously administered therapeutic adenoviruses
Abstract
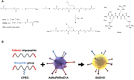
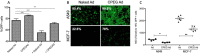
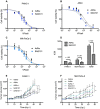
Oncolytic adenoviruses are used as agents for the treatment of cancer. However, their potential is limited due to the high seroprevalence of anti-adenovirus neutralizing antibodies (nAbs) within the population and the rapid liver sequestration when systemically administered. To overcome these challenges, we explored using nanoparticle formulation to boost the efficacy of systemic oncolytic adenovirus administration. Methods: Adenovirus were conjugated with PEGylated oligopeptide-modified poly(β-amino ester)s (OM-pBAEs). The resulting coated viral formulation was characterized in terms of surface charge, size, aggregation state and morphology and tested for anti-adenovirus nAbs evasion and activity in cancer cells. In vivo pharmacokinetics, biodistribution, tumor targeting, and immunogenicity studies were performed. The antitumor efficacy of the oncolytic adenovirus AdNuPARmE1A coated with OM-pBAEs (SAG101) in the presence of nAbs was evaluated in pancreatic ductal adenocarcinoma (PDAC) mouse models. Toxicity of the coated formulation was analyzed in vivo in immunocompetent mice. Results: OM-pBAEs conjugated to adenovirus and generated discrete nanoparticles with a neutral charge and an optimal size. The polymeric coating with the reporter AdGFPLuc (CPEG) showed enhanced transduction and evasion of antibody neutralization in vitro. Moreover, systemic intravenous administration of the formulation showed improved blood circulation and reduced liver sequestration, substantially avoiding activation of nAb production. OM-pBAEs coating of the oncolytic adenovirus AdNuPARmE1A (SAG101) improved its oncolytic activity in vitro and enhanced antitumor efficacy in PDAC mouse models. The coated formulation protected virions from neutralization by nAbs, as antitumor efficacy was preserved in their presence but was completely lost in mice that received the non-formulated AdNuPARmE1A. Finally, coated-AdNuPARmE1A showed reduced toxicity when high doses of the formulation were administered. Conclusions: The developed technology represents a promising improvement for future clinical cancer therapy using oncolytic adenoviruses.
Keywords: oncolytic adenovirus; pancreatic cancer; poly(β-amino ester)s; polymer-coated viral vectors; systemic delivery.
© The author(s).
Conflict of interest statement
Competing Interests: SAG-101 is a therapeutic product under development by Sagetis-Biotech. A Cascante, MA Lázaro, C Castells, C Fornaguera, and S Borrós were hired by this company after all experimental work had been performed. C. Fillat is member of the advisory board of Sagetis-Biotech.
Figures




References
-
- Twumasi-Boateng K, Pettigrew JL, Kwok YYE, Bell JC, Nelson BH. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat Rev Cancer. 2018;18:419–32. - PubMed
-
- Pilankatta R, Chawla T, Khanna N, Swaminathan S. The prevalence of antibodies to adenovirus serotype 5 in an adult Indian population and implications for adenovirus vector vaccines. J Med Virol. 2010;82:407–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

