Genome-Wide Sequencing for Unexplained Developmental Disabilities or Multiple Congenital Anomalies: A Health Technology Assessment
- PMID: 32194879
- PMCID: PMC7080457
Genome-Wide Sequencing for Unexplained Developmental Disabilities or Multiple Congenital Anomalies: A Health Technology Assessment
Abstract
Background: People with unexplained developmental disabilities or multiple congenital anomalies might have had many biochemical, metabolic, and genetic tests for a period of years without receiving a diagnosis. A genetic diagnosis can help these people and their families better understand their condition and may help them to connect with others who have the same condition. Ontario Health (Quality), in collaboration with the Canadian Agency for Drugs and Technologies in Health (CADTH) conducted a health technology assessment about the use of genome-wide sequencing for patients with unexplained developmental disabilities or multiple congenital anomalies. Ontario Health (Quality) evaluated the effectiveness, cost-effectiveness, and budget impact of publicly funding genome-wide sequencing. We also conducted interviews with patients and examined the quantitative evidence of preferences and values literature to better understand the patient preferences and values for these tests.
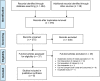
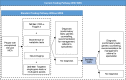
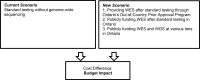
Methods: Ontario Health (Quality) performed a systematic literature search of the clinical evidence. We assessed the risk of bias of each included study using the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS) and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We also performed a search of the quantitative evidence and undertook direct patient engagement to ascertain patient preferences for genetic testing for unexplained developmental disabilities or multiple congenital anomalies. CADTH performed a review of qualitative literature about patient perspectives and experiences, and a review of ethical issues.Ontario Health (Quality) performed an economic literature review of genome-wide sequencing in people with unexplained developmental disabilities or multiple congenital anomalies. Although we found eight published cost-effectiveness studies, none completely addressed our research question. Therefore, we conducted a primary economic evaluation using a discrete event simulation model. Owing to its high cost and early stage of clinical implementation, whole exome sequencing is primarily used for people who do not have a diagnosis from standard testing (referred to here as whole exome sequencing after standard testing; standard testing includes chromosomal microarray and targeted single-gene tests or gene panels). Therefore, in our first analysis, we evaluated the cost-effectiveness of whole exome sequencing after standard testing versus standard testing alone. In our second analysis, we explored the cost-effectiveness of whole exome and whole genome sequencing used at various times in the diagnostic pathway (e.g., first tier, second tier, after standard testing) versus standard testing. We also estimated the budget impact of publicly funding genome-wide sequencing in Ontario for the next 5 years.
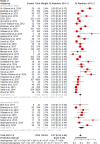
Results: Forty-four studies were included in the clinical evidence review. The overall diagnostic yield of genome-wide sequencing for people with unexplained development disability and multiple congenital anomalies was 37%, but we are very uncertain about this estimate (GRADE: Very Low). Compared with standard genetic testing of chromosomal microarray and targeted single-gene tests or gene panels, genome-wide sequencing could have a higher diagnostic yield (GRADE: Low). As well, for some who are tested, genome-wide sequencing prompts some changes to medications, treatments, and referrals to specialists (GRADE: Very Low).Whole exome sequencing after standard testing cost an additional $3,261 per patient but was more effective than standard testing alone. For every 1,000 persons tested, using whole exome sequencing after standard testing would lead to an additional 240 persons with a molecular diagnosis, 272 persons with any positive finding, and 46 persons with active treatment change (modifications to medications, procedures, or treatment). The resulting incremental cost-effectiveness ratios (ICERs) were $13,591 per additional molecular diagnosis. The use of genome-wide sequencing early in the diagnostic pathway (e.g., as a first- or second-tier test) can save on costs and improve diagnostic yields over those of standard testing. Results remained robust when parameters and assumptions were varied.Our budget impact analysis showed that, if whole exome sequencing after standard testing continues to be funded through Ontario's Out-of-Country Prior Approval Program, its budget impact would range from $4 to $5 million in years 1 to 5. If whole exome sequencing becomes publicly funded in Ontario (not through the Out-of-Country Prior Approval Program), the budget impact would be about $9 million yearly. We also found that using whole exome sequencing as a second-tier test would lead to cost savings ($3.4 million per 1,000 persons tested yearly).Participants demonstrated consistent motivations for and expectations of obtaining a diagnosis for unexplained developmental delay or congenital anomalies through genome-wide sequencing. Patients and families greatly value the support and information they receive through genetic counselling when considering genome-wide sequencing and learning of a diagnosis.
Conclusions: Genome-wide sequencing could have a higher diagnostic yield than standard testing for people with unexplained developmental disabilities or multiple congenital anomalies. Genome-wide sequencing can also prompt some changes to medications, treatments, and referrals to specialists for some people tested; however, we are very uncertain about this. Genome-wide sequencing could be a cost-effective strategy when used after standard testing to diagnose people with unexplained developmental disabilities or multiple congenital anomalies. It could also lead to cost savings when used earlier in the diagnostic pathway. Patients and families consistently noted a benefit from seeking a diagnosis through genetic testing.
Copyright © Queen's Printer for Ontario, 2020.
Figures




















References
-
- O'Byrne JJ, Lynch SA, Treacy EP, King MD, Betts DR, Mayne PD, et al. Unexplained developmental delay/learning disability: guidelines for best practice protocol for first line assessment and genetic/metabolic/radiological investigations. Ir J Med Sci. 2016;185(1):241–8. - PubMed
-
- Arim R. A profile of persons with disabilities among Canadians aged 15 years or older, 2012 [Internet]. Ottawa (ON): Statistics Canada; 2015. [cited 2018 October 10]. Available from: https://www150.statcan.gc.ca/n1/pub/89–654-x/89–654-x2015001-eng.htm#a12
-
- Congenital anomalies in Canada [Internet]. Ottawa (ON): Public Health Agency of Canada; 2017. [updated Nov 30, 2017; cited 2018 Oct 10]. Available from: https://infobase.phac-aspc.gc.ca/congenital-anomalies/index
-
- Centers for Disease Control and Prevention. Data & statistics on birth defects [Internet]. Atlanta (GA): The Centers; 2018. [updated Apr 30, 2018; cited 2018 Oct 10]. Available from: https://www.cdc.gov/ncbddd/birthdefects/data.html
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
