Optimizing COPD treatment in patients with lung- or head and neck cancer does not improve quality of life - a randomized, pilot, clinical trial
- PMID: 32194927
- PMCID: PMC7067155
- DOI: 10.1080/20018525.2020.1731277
Optimizing COPD treatment in patients with lung- or head and neck cancer does not improve quality of life - a randomized, pilot, clinical trial
Abstract
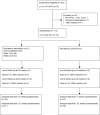
Background: Chronic obstructive pulmonary disease (COPD) is a common comorbidity in patients with lung and head- and neck cancer. Patients with lung cancer who also suffer from COPD have a worse prognosis than patients with lung cancer and no COPD. It has previously been shown that diagnosis and treatment of concomitant COPD in patients with newly diagnosed lung- or head and neck cancer need optimization. In this randomized, controlled trial we aimed to assess if intervention directed at improving treatment for COPD in these patients improved health-related quality of life (QoL). Methods: During 2014, we randomized 114 patients referred for oncological treatment at a large university hospital in the Capital Region of Denmark, to either usual care or intervention regarding concomitant COPD. The intervention consisted of two visits in an out-patient clinic established at the oncological department and staffed with a pulmonary physician. At baseline, week 13 and week 25, all patients filled out the cancer- and COPD-specific QoL questionnaires CAT and EORTC, respectively. The primary outcome was change in CAT-score between control- and intervention group. The secondary outcome was change in EORTC. Results: There was no change in CAT-score by week 13 or 25 between the groups. For the EORTC there was a statistically significant improvement only in the fatigue domain at week 13 (p = 0.03), but not at week 25. There was a trend towards less dyspnea in the intervention group at week 13, measured by EORTC (p = 0.07). Mortality by week 25 was similar in both groups. Conclusion: In this population of severely ill cancer patients, we did not find that this intervention, focusing on inhaled COPD medication, for the management of COPD had any convincing positive impact on the patients' perceived quality of life compared with usual care. Further studies are needed.
Keywords: COPD; comorbidity; lung cancer.
© 2020 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
References
-
- [cited 2019. April 4]. Available from: https://goldcopd.org/.
-
- Chaukar DA, Walvekar RR, Das AK, et al. Quality of life in head and neck cancer survivors: a cross-sectional survey. Am J Otolaryngol. 2009;30(3):176–180. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous