Generation of a three-dimensional collagen scaffold-based model of the human endometrium
- PMID: 32194932
- PMCID: PMC7061944
- DOI: 10.1098/rsfs.2019.0079
Generation of a three-dimensional collagen scaffold-based model of the human endometrium
Abstract
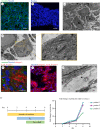
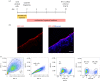
The endometrium is the secretory lining of the uterus that undergoes dynamic changes throughout the menstrual cycle in preparation for implantation and a pregnancy. Recently, endometrial organoids (EO) were established to study the glandular epithelium. We have built upon this advance and developed a multi-cellular model containing both endometrial stromal and epithelial cells. We use porous collagen scaffolds produced with controlled lyophilization to direct cellular organization, integrating organoids with primary isolates of stromal cells. The internal pore structure of the scaffold was optimized for stromal cell culture in a systematic study, finding an optimal average pore size of 101 µm. EO seeded organize to form a luminal-like epithelial layer, on the surface of the scaffold. The cells polarize with their apical surface carrying microvilli and cilia that face the pore cavities and their basal surface attaching to the scaffold with the formation of extracellular matrix proteins. Both cell types are hormone responsive on the scaffold, with hormone stimulation resulting in epithelial differentiation and stromal decidualization.
Keywords: co-culture; collagen scaffolds; endometrium; organoids.
© 2020 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
