Establishment of patient-derived tumor xenograft models of mucinous ovarian cancer
- PMID: 32195028
- PMCID: PMC7061742
Establishment of patient-derived tumor xenograft models of mucinous ovarian cancer
Abstract
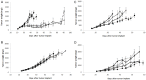
Mucinous ovarian carcinoma (mEOC) represents a rare subtype of epithelial ovarian cancer, accounting for 3-4% of all ovarian carcinomas. The rarity of this tumor type renders both the preclinical and clinical research compelling. Very few preclinical in vitro and in vivo models exist. We here report the molecular, metabolic and pharmacological characterization of two patient derived xenografts (PDXs) from mEOC, recently obtained in our laboratory. These PDXs maintain the histological and molecular characteristics of the patient's tumors they derived from, including a wild type TP53. Gene expression analysis and metabolomics profile suggest that they differ from high grade serous/endometrioid ovarian carcinoma PDXs. The pharmacological characterization was undertaken testing the in vivo antitumor activity of both cytotoxic agents (cisplatin, paclitaxel, yondelis, oxaliplatin and 5-fluorouracile) and targeted agents (bevacizumab and lapatinib). These newly established mucinous PDXs do recapitulate mEOC and will be of value in the preclinical development of possible new therapeutic strategies for this tumor type.
Keywords: Patient-derived xenografts; chemotherapy; mucinous ovarian cancer.
AJCR Copyright © 2020.
Conflict of interest statement
None.
Figures


References
-
- Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–4. - PubMed
-
- Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry JP, Scolyer RA, Smith AN, Bali A, Vanden Bergh P, Baron-Hay S, Scott C, Fink D, Hacker NF, Sutherland RL, O’Brien PM. A distinct molecular profile associated with mucinous epithelial ovarian cancer. Br J Cancer. 2006;94:904–13. - PMC - PubMed
-
- Leary AF, Quinn M, Fujiwara K, Coleman RL, Kohn E, Sugiyama T, Glasspool R, Ray-Coquard I, Colombo N, Bacon M, Zeimet A, Westermann A, Gomez-Garcia E, Provencher D, Welch S, Small W, Millan D, Okamoto A, Stuart G, Ochiai K Participants of the Fifth Ovarian Cancer Consensus Conference. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup (GCIG): clinical trial design for rare ovarian tumours. Ann Oncol. 2017;28:718–726. - PMC - PubMed
-
- Perren TJ. Mucinous epithelial ovarian carcinoma. Ann Oncol. 2016;27(Suppl 1):i53–i57. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
