The HIPPO pathway in gynecological malignancies
- PMID: 32195031
- PMCID: PMC7061741
The HIPPO pathway in gynecological malignancies
Abstract
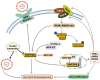
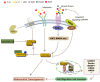
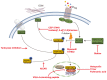
The Hippo pathway has been initially discovered by screening genes that regulate organ size in Drosophila. Recent studies have highlighted the role of the Hippo pathway in controlling organ size, tissue homeostasis and regeneration, and signaling dysregulation, especially the overactivation of the transcriptional coactivator YAP/TAZ, which leads to uncontrolled cell growth and malignant transformation. The core components of the Hippo pathway may initiate tumorigenesis by inducing tumor stem cells and proliferation, ultimately leading to metastasis and drug resistance, which occurs extensively in gynecological malignancies, including cervical cancer, ovarian cancer, and endometrial cancer. In this review, we attempt to systematically summarize recent progress in our understanding of the mechanism of Hippo pathway regulation in tumorigenesis and the mechanisms that underlie alterations during gynecological malignancies, as well as new therapeutic strategies.
Keywords: Hippo pathway; YAP/TAZ; cervical cancer; endometrial cancer; ovarian cancer; therapeutic strategies; tumorigenesis.
AJCR Copyright © 2020.
Conflict of interest statement
None.
Figures

References
-
- Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D, Hariharan IK. Salvador promotes both cell cycle exit and apoptosis in drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. - PubMed
-
- Maugeri-Sacca M, De Maria R. Hippo pathway and breast cancer stem cells. Crit Rev Oncol Hematol. 2016;99:115–122. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
