Molecular Recognition by Zn(II)-Capped Dynamic Foldamers
- PMID: 32195074
- PMCID: PMC7080544
- DOI: 10.1002/open.201900362
Molecular Recognition by Zn(II)-Capped Dynamic Foldamers
Abstract
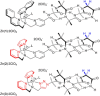
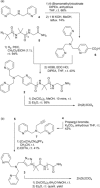
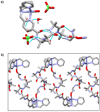
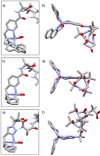
Two α-aminoisobutyric acid (Aib) foldamers bearing Zn(II)-chelating N-termini have been synthesized and compared with a reported Aib foldamer that has a bis(quinolinyl)/mono(pyridyl) cap (BQPA group). Replacement of the quinolinyl arms of the BQPA-capped foldamer with pyridyl gave a BPPA-capped foldamer, then further replacement of the linking pyridyl with a 1,2,3-triazole gave a BPTA-capped foldamer. Their ability to relay chiral information from carboxylate bound to Zn(II) at the N-terminus to a glycinamide-based NMR reporter of conformational preference at the C-terminus was measured. The importance of the quinolinyl arms became readily apparent, as the foldamers with pyridyl arms were unable to report on the presence of chiral carboxylate in acetonitrile. Low solubility, X-ray crystallography and 1H NMR spectroscopy suggested that interfoldamer interactions inhibited carboxylate binding. However changing solvent to methanol revealed that the end-to-end relay of chiral information could be observed for the Zn(II) complex of the BPTA-capped foldamer at low temperature.
Keywords: molecular recognition; peptides; receptors; self-assembly; supramolecular chemistry.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures

References
-
- Feng G., Mareque-Rivas J. C., de Rosales R. T. M., Williams N. H. J. Am. Chem. Soc. 2005, 127, 13470–13471. - PubMed
-
- None
-
- J. M. Castagnetto, J. W. Canary, Chem. Commun 1998, 203–204;
-
- Dai Z., Canary J. W., New J. Chem. 2007, 31, 1708–1718.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

