Differentiation Potential of Nestin (+) and Nestin (-) Cells Derived from Human Bone Marrow Mesenchymal Stem Cells into Functional Insulin Producing Cells
- PMID: 32195201
- PMCID: PMC7073261
- DOI: 10.22088/IJMCM.BUMS.8.1.1
Differentiation Potential of Nestin (+) and Nestin (-) Cells Derived from Human Bone Marrow Mesenchymal Stem Cells into Functional Insulin Producing Cells
Abstract
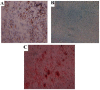
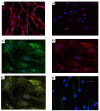
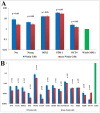
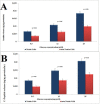
The feasibility of isolating and manipulating mesenchymal stem cells (MSCs) from human patients provides hope for curing numerous diseases and disorders. Recent phenotypic analysis has shown heterogeneity of MSCs. Nestin progenitor cell is a subpopulation within MSCs which plays a role in pancreas regeneration during embryogenesis. This study aimed to separate nestin (+) cells from human bone marrow MSCs, and differentiate these cells into functional insulin producing cells (IPCs) compared with nestin (-) cells. Manual magnetic separation was performed to obtain nestin (+) cells from MSCs. Approximately 91±3.3% of nestin (+) cells were positive for anti-nestin antibody. Pluripotent genes were overexpressed in nestin (+) cells compared with nestin (-) cells as revealed by quantitative real time-PCR (qRT-PCR). Following in vitro differentiation, flow cytometric analysis showed that 2.7±0.5% of differentiated nestin (+) cells were positive for anti-insulin antibody in comparison with 0.08±0.02% of nestin (-) cells. QRT-PCR showed higher expression of insulin and other endocrine genes in comparison with nestin (-) cells. While immunofluorescence technique showed the presence of insulin and C-peptide granules in nestin (+) cells. Therefore, our results introduced nestin (+) cells as a pluripotent subpopulation within human MSCs which is capable to differentiate and produce functional IPCs.
Keywords: Human bone marrow derived mesenchymal stem cells; diabetes mellitus; insulin producing cells; mesenchymal stem cells; real time-quantitative PCR.
Figures

References
-
- Hirshberg B, Rother KI, Digon BJ, 3rd , et al. Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: the National Institutes of Health experience. Diabetes Care. 2003;26:3288–95. - PubMed
-
- Sun Y, Chen L, Hou XG, et al. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J (Engl) 2007;120:771–6. - PubMed
-
- Karnieli O, Izhar-Prato Y, Bulvik S, et al. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837–44. - PubMed
LinkOut - more resources
Full Text Sources
