Deep Learning for Cardiac Image Segmentation: A Review
- PMID: 32195270
- PMCID: PMC7066212
- DOI: 10.3389/fcvm.2020.00025
Deep Learning for Cardiac Image Segmentation: A Review
Abstract
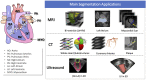
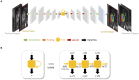
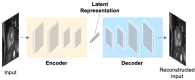
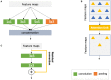
Deep learning has become the most widely used approach for cardiac image segmentation in recent years. In this paper, we provide a review of over 100 cardiac image segmentation papers using deep learning, which covers common imaging modalities including magnetic resonance imaging (MRI), computed tomography (CT), and ultrasound and major anatomical structures of interest (ventricles, atria, and vessels). In addition, a summary of publicly available cardiac image datasets and code repositories are included to provide a base for encouraging reproducible research. Finally, we discuss the challenges and limitations with current deep learning-based approaches (scarcity of labels, model generalizability across different domains, interpretability) and suggest potential directions for future research.
Keywords: CT; MRI; artificial intelligence; cardiac image analysis; cardiac image segmentation; deep learning; neural networks; ultrasound.
Copyright © 2020 Chen, Qin, Qiu, Tarroni, Duan, Bai and Rueckert.
Figures




References
-
- Tavakoli V, Amini AA. A survey of shaped-based registration and segmentation techniques for cardiac images. Comput Vis Image Understand. (2013) 117:966–89. 10.1016/j.cviu.2012.11.017 - DOI
-
- Greenspan H, Van Ginneken B, Summers RM. Guest editorial deep learning in medical imaging: overview and future promise of an exciting new technique. IEEE Trans Med Imaging. (2016) 35:1153–9. 10.1109/TMI.2016.2553401 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

