Laboratory studies for paroxysmal nocturnal hemoglobinuria, with emphasis on flow cytometry
- PMID: 32195308
- PMCID: PMC7078534
- DOI: 10.1016/j.plabm.2020.e00158
Laboratory studies for paroxysmal nocturnal hemoglobinuria, with emphasis on flow cytometry
Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired clonal hematopoietic stem cell disorder caused by somatic mutations in the PIG-A gene, leading to the production of blood cells with absent or decreased expression of glycosylphosphatidylinositol-anchored proteins, including CD55 and CD59. Clinically, PNH is classified into three variants: classic (hemolytic), in the setting of another specified bone marrow disorder (such as aplastic anemia or myelodysplastic syndrome) and subclinical (asymptomatic). PNH testing is recommended for patients with intravascular hemolysis, acquired bone marrow failure syndromes and thrombosis with unusual features. Despite the availability of consensus guidelines for PNH diagnosis and monitoring, there are still discrepancies on how PNH tests are carried out, and these technical variations may lead to an incorrect diagnosis. Herein, we provide a brief historical overview of PNH, focusing on the laboratory tests available and on the current recommendations for PNH diagnosis and monitoring based in flow cytometry.
Keywords: Bone marrow failure syndromes; Flow cytometry; Glycosylphosphatidylinositol; Intravascular hemolysis; PNH; Paroxysmal nocturnal hemoglobinuria.
© 2020 Published by Elsevier B.V.
Conflict of interest statement
The author declares that there is no conflict of interest regarding the publication of this paper.
Figures
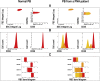
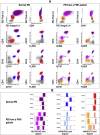
References
-
- Devalet B., Mullier F., Chatelain B., Dogné J.-M., Chatelain C. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur. J. Haematol. 2015;95:190–198. - PubMed
-
- Brodsky R.A. Complement in hemolytic anemia. Blood. 2015;126:2459–2465. - PubMed
-
- Schröder-Braunstein J., Kirschfink M. Complement deficiencies and dysregulation: pathophysiological consequences, modern analysis, and clinical management. Mol. Immunol. 2019;114:299–311. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

