miR-22 inhibition reduces hepatic steatosis via FGF21 and FGFR1 induction
- PMID: 32195457
- PMCID: PMC7078383
- DOI: 10.1016/j.jhepr.2020.100093
miR-22 inhibition reduces hepatic steatosis via FGF21 and FGFR1 induction
Abstract
Background & aims: Metabolism supports cell proliferation and growth. Surprisingly, the tumor suppressor miR-22 is induced by metabolic stimulators like bile acids. Thus, this study examines whether miR-22 could be a metabolic silencer.
Methods: The relationship between miR-22 and the expression of fibroblast growth factor 21 (FGF21) and its receptor FGFR1 was studied in cells and fatty livers obtained from patients and mouse models. We evaluated the effect of an miR-22 inhibitor alone and in combination with obeticholic acid (OCA) for the treatment of steatosis.
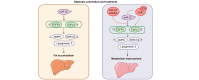
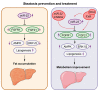
Results: The levels of miR-22 were inversely correlated with those of FGF21, FGFR1, and PGC1α in human and mouse fatty livers, suggesting that hepatic miR-22 acts as a metabolic silencer. Indeed, miR-22 reduced FGFR1 by direct targeting and decreased FGF21 by reducing the recruitment of PPARα and PGC1α to their binding motifs. In contrast, an miR-22 inhibitor increases hepatic FGF21 and FGFR1, leading to AMPK and ERK1/2 activation, which was effective in treating alcoholic steatosis in mouse models. The farnesoid x receptor-agonist OCA induced FGF21 and FGFR1, as well as their inhibitor miR-22. An miR-22 inhibitor and OCA were effective in treating diet-induced steatosis, both alone and in combination. The combined treatment was the most effective at improving insulin sensitivity, releasing glucagon-like peptide 1, and reducing hepatic triglyceride in obese mice.
Conclusion: The simultaneous induction of miR-22, FGF21 and FGFR1 by metabolic stimulators may maintain FGF21 homeostasis and restrict ERK1/2 activation. Reducing miR-22 enhances hepatic FGF21 and activates AMPK, which could be a novel approach to treat steatosis and insulin resistance.
Lay summary: This study examines the metabolic role of a tumor suppressor, miR-22, that can be induced by metabolic stimulators such as bile acids. Our novel data revealed that the metabolic silencing effect of miR-22 occurs as a result of reductions in metabolic stimulators, which likely contribute to the development of fatty liver. Consistent with this finding, an miR-22 inhibitor effectively reversed both alcohol- and diet-induced fatty liver; miR-22 inhibition is a promising therapeutic option which could be used in combination with obeticholic acid.
Keywords: 3'-UTR, 3' untranslated region; ALP, alkaline phosphatase; ALT, alanine aminotransferase; CD, control diet; FGF21, fibroblast growth factor 21; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide; HDAC, histone deacetylase; ITT, insulin tolerance test; LPS, lipopolysaccharide; NPCs, non-parenchymal cells; OCA, obeticholic acid; PFUs, plaque-forming units; PGC1α, PPAR-activated receptor-γ coactivator-1α; PHHs, primary human hepatocytes; PPREs, peroxisome proliferative-response elements; RARβ, retinoic acid receptor β; RT-PCR, reverse transcription PCR; SIRT1, sirtuin 1; Steatosis; WD, Western diet; alcoholic steatosis; insulin sensitivity; metabolic syndrome; non-alcoholic steatohepatitis; obeticholic acid.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures







References
-
- Neely L.A., Patel S., Garver J., Gallo M., Hackett M., McLaughlin S. A single-molecule method for the quantitation of microRNA gene expression. Nat Methods. 2006;3:41–46. - PubMed
-
- Xiong J., Yu D., Wei N., Fu H., Cai T., Huang Y. An estrogen receptor alpha suppressor, microRNA-22, is downregulated in estrogen receptor alpha-positive human breast cancer cell lines and clinical samples. FEBS J. 2010;277:1684–1694. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

