Placebo Responses Among Men With Erectile Dysfunction Enrolled in Phosphodiesterase 5 Inhibitor Trials: A Systematic Review and Meta-analysis
- PMID: 32196105
- PMCID: PMC7084170
- DOI: 10.1001/jamanetworkopen.2020.1423
Placebo Responses Among Men With Erectile Dysfunction Enrolled in Phosphodiesterase 5 Inhibitor Trials: A Systematic Review and Meta-analysis
Abstract
Importance: Placebo responses in the treatment of erectile dysfunction (ED) are poorly described in the literature to date.
Objective: To quantify the association of placebo with ED outcomes among men enrolled in placebo-controlled, phosphodiesterase 5 inhibitor (PDE5I) trials.
Data sources: For this systematic review and meta-analysis, a database search was conducted to identify double-blind, placebo-controlled studies using PDE5Is for the treatment of ED published from January 1, 1998, to December 31, 2018, within MEDLINE, Embase, Cochrane Library, and Web of Science. Only articles published in the English language were included.
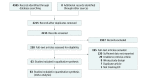
Study selection: Double-blind, placebo-controlled randomized clinical trials of PDE5Is for ED were included. Studies were excluded if they did not provide distribution measures for statistical analysis. Study selection review assessments were conducted by 2 independent investigators. A total of 2215 studies were identified from the database search, and after review, 63 studies that included 12 564 men were analyzed.
Data extraction and synthesis: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in abstracting data and assessing validity. Data were extracted from published reports by 2 independent reviewers. Quality assessment was performed using the Jadad scale. Data were pooled using a random-effects model.
Main outcomes and measures: The main outcome was improvement in the erectile function domain of the International Index of Erectile Function questionnaire in the placebo arm of the included studies. Effect size was reported as bias-corrected standardized mean difference (Hedges g). The hypothesis was formulated before data extraction.
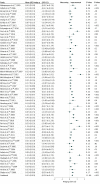
Results: A total of 63 studies that included 12 564 men (mean [SD] age, 55 [7] years; age range, 36-68 years) were included. Erectile function was significantly improved among participants in the placebo arm, with a small to moderate effect size (Hedges g [SE], 0.35 [0.03]; P < .001). Placebo effect size was larger among participants with ED associated with posttraumatic stress disorder (Hedges g [SE], 0.78 [0.32]; P = .02) compared with the overall analysis. No significant difference was found between placebo and PDE5Is for ED after prostate surgery or radiotherapy (Hedges g [SE], 0.30 [0.17]; P = .08).
Conclusions and relevance: In this study, placebo was associated with improvement of ED, especially among men with ED-related posttraumatic stress disorder. No difference was found between placebo and PDE5I among men treated for ED after prostate surgery.
Conflict of interest statement
Figures
Comment in
-
Re: Placebo Responses among Men with Erectile Dysfunction Enrolled in Phosphodiesterase 5 Inhibitor Trials: A Systematic Review and Meta-Analysis.J Urol. 2020 Sep;204(3):600. doi: 10.1097/JU.0000000000001167.02. Epub 2020 Jun 26. J Urol. 2020. PMID: 32586193 No abstract available.
Similar articles
-
Assessment of Combination Therapies vs Monotherapy for Erectile Dysfunction: A Systematic Review and Meta-analysis.JAMA Netw Open. 2021 Feb 1;4(2):e2036337. doi: 10.1001/jamanetworkopen.2020.36337. JAMA Netw Open. 2021. PMID: 33599772 Free PMC article.
-
Combination Therapy with Alpha-blocker and Phosphodiesterase-5 Inhibitor for Improving Lower Urinary Tract Symptoms and Erectile Dysfunction in Comparison with Monotherapy: A Systematic Review and Meta-analysis.Eur Urol Focus. 2020 May 15;6(3):537-558. doi: 10.1016/j.euf.2019.05.007. Epub 2019 May 25. Eur Urol Focus. 2020. PMID: 31133414
-
Tadalafil once daily in men with erectile dysfunction: an integrated analysis of data obtained from 1913 patients from six randomized, double-blind, placebo-controlled, clinical studies.Eur Urol. 2014 Feb;65(2):455-64. doi: 10.1016/j.eururo.2013.09.037. Epub 2013 Oct 2. Eur Urol. 2014. PMID: 24119319
-
Effects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia and on erectile dysfunction in sexually active men with both conditions: analyses of pooled data from four randomized, placebo-controlled tadalafil clinical studies.J Sex Med. 2013 Aug;10(8):2044-52. doi: 10.1111/jsm.12212. Epub 2013 Jun 19. J Sex Med. 2013. PMID: 23782459 Clinical Trial.
-
Efficacy and Safety of a Fixed-Dose Combination Therapy of Tamsulosin and Tadalafil for Patients With Lower Urinary Tract Symptoms and Erectile Dysfunction: Results of a Randomized, Double-Blinded, Active-Controlled Trial.J Sex Med. 2017 Aug;14(8):1018-1027. doi: 10.1016/j.jsxm.2017.06.006. J Sex Med. 2017. PMID: 28760246 Clinical Trial.
Cited by
-
Efficacy and Safety of Avanafil in Chinese Subjects With Erectile Dysfunction: A Multi-Center, Randomized, Double-Blinded, Placebo-Controlled Phase III Clinical Trial.Sex Med. 2021 Jun;9(3):100337. doi: 10.1016/j.esxm.2021.100337. Epub 2021 Mar 6. Sex Med. 2021. PMID: 33685839 Free PMC article.
-
Platelet-rich plasma therapy in erectile dysfunction and Peyronie's disease: a systematic review of the literature.World J Urol. 2024 May 29;42(1):359. doi: 10.1007/s00345-024-05065-3. World J Urol. 2024. PMID: 38811395 Free PMC article.
-
Effect of standardized root extract of ashwagandha (Withania somnifera) on well-being and sexual performance in adult males: A randomized controlled trial.Health Sci Rep. 2022 Jul 20;5(4):e741. doi: 10.1002/hsr2.741. eCollection 2022 Jul. Health Sci Rep. 2022. PMID: 35873404 Free PMC article.
-
The placebo and nocebo effects in functional urology.Nat Rev Urol. 2022 Mar;19(3):171-189. doi: 10.1038/s41585-021-00545-2. Epub 2021 Dec 23. Nat Rev Urol. 2022. PMID: 34949831 Review.
-
Assessment of Combination Therapies vs Monotherapy for Erectile Dysfunction: A Systematic Review and Meta-analysis.JAMA Netw Open. 2021 Feb 1;4(2):e2036337. doi: 10.1001/jamanetworkopen.2020.36337. JAMA Netw Open. 2021. PMID: 33599772 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

